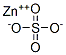
Zinc sulphate synthesis
- Product Name:Zinc sulphate
- CAS Number:7733-02-0
- Molecular formula:ZnSO4
- Molecular Weight:161.45
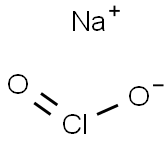
7758-19-2
300 suppliers
$37.79/250ML
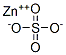
7733-02-0
491 suppliers
$7.00/5g
Yield:-
Reaction Conditions:
with sodium hydroxide
Steps:
Sample 116
Sample 116
An aqueous composition was prepared as follows.
A glass beaker was placed on a scale, and then, 4.5 g (12.44 mmol) of 25% sodium chlorite solution was placed in the beaker.
Next, 94.3 ml of deionized water was added to the beaker.
After adding the water, a magnetic stirrer was added to the beaker and the contents of the beaker were stirred.
Next, 1 g (12.5 mmol) of the 50% sodium hydroxide solution was added to the beaker, and the contents of the beaker were further stirred.
Next, 0.27 g (1.7 mmol) of anhydrous zinc sulfate (obtained from VWR) was added to the beaker and the contents were mixed to produce an aqueous composition.
The composition included white crystals at the time of preparation.
References:
US2018/179058,2018,A1
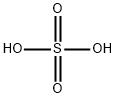
7664-93-9
0 suppliers
$23.30/1090731000
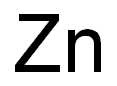
7440-66-6
0 suppliers
$14.00/5g
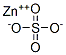
7733-02-0
491 suppliers
$7.00/5g