| Identification | Back Directory | [Name]
INSULIN | [CAS]
11061-68-0 | [Synonyms]
D03230
Umulin
INSULIN
novolin
Humulin
humuline
Oral-lyn
Penfil R
ORMD 0801
velosulin
rh-Insulin
Ultraphane
humaninsulin
Velosulin HM
INSULIN PORC
Humulin (tn)
INSULIN HUMAN
Insulin Human RS
INSULIN (PORCINE)
Insulin(human)?, BR
INSULIN SODIUM SALT
Insulin (human) CRS
Insulin (human) reco
insulin solution human
Human Insulin, Solution
HUMANINSULIN(SYNTHESIS)
INSULIN BOVINE PANCREAS
insulinhuman(synthesis)
Insulin Human (100 mg)
INSULIN, RECOMBINANT HUMAN
Insulin sodium salt human
Insulin human (biosynthesis)
Insulin, Zinc, Human, Recomb.
RecoMbinant HuMan Insulin EP/USP
Insulin human (biosynthesis) (jan)
Insulin, Zinc, Human, Recombinant,
Insulin human (genetical recombination)
MIDAZOLAM MALEATE SALT. DEA SCH IV ITEM
Insulin solution from bovine pancreas
Insulin, recombinant human, min. 27.5 IU/mg
INSULIN HUMAN, RECOMBINANT EXPRESSED IN YEAST
Insulin, Zinc, Human, Recombinant, P. pastoris
Insulin human (genetical recombination) (jp15)
Insulin Human (100 mg) (COLD SHIPMENT REQUIRED)
INSULIN HUMAN, RECOMBINANT EXPRESSED IN E. COLI
High Molecular Weight Insulin Human (8.4 mg) (COLD SHIPMENT REQUIRED) | [EINECS(EC#)]
234-291-2 | [Molecular Formula]
C257H383N65O77S6 | [MDL Number]
MFCD00131380 |
| Chemical Properties | Back Directory | [Appearance]
White or almost white powder. | [Melting point ]
>200°C (dec.) | [storage temp. ]
2-8°C
| [solubility ]
acidified water, pH 2.0: 2 mg/mL
| [form ]
solution
| [color ]
White | [Water Solubility ]
Soluble up to 10mg/ml in pH <3 or in the presence of surfactants. Adjusting pH with a volatile acid (such as formic acid) prior to dry down will allow the product to be re-dissolved in water. |
| Questions And Answer | Back Directory | [Gene, mRNA, and precursor]
The human preproINS gene, INS, is located at 11p15.5
and consists of three exons. In mice, it is located on chromosome 7. There are many regulatory elements, such as
A-box, GG box, and the cAMP response element, in the
promoter region. The coding region of human and tilapia
preproINS mRNAs comprises 330 and 339 bases, respectively. In humans, INS forms a gene cluster with IGF2 and H19, which is a gene for a long noncoding
RNA.
 | [Regulation of synthesis]
INS gene transcription is stimulated by glucose in
mammals, and glucose treatment results in a 2- to
5-fold elevation within 60–90min. Long-term exposure
to physiological concentrations of estradiol-17β increases
the β-cell content, INS gene expression, and INS release in mice. Fasting reduces the expression levels of two INS
genes in the trout. | [Receptors]
INS receptors (INSRs) are heterotetrameric glycoproteins containing many phosphorylated or glycosylated
sites. INS binds to the two extracellular α-subunits linked
by a disulfide bond. The two β-subunits are
connected to the α-subunits by a disulfide bond and contain a tyrosine kinase domain in their intracellular
region. Human INSR is encoded by a single gene,
INSR, location 19p13.3-p13.2, comprising 22 exons.INSRencodes 1382 aa residues of the ISNR precursor, which contains a signal peptide and α- and β-subunits.
Human INSR generates two alternative splicing variants
(INSR-A and -B) that differ at the carboxyl terminus of their
α-subunit. INSR-A lacks the terminus encoded by exon 11.
INSR-B has not been detected in the chicken or Xenopus. There are three types of hybrid receptors among these
INSRs and the IGF-I receptor (IGF-IR), and their expression ratio influences the INS action at the target
organs. Dysregulation of the INSR-A/INSR-B ratio is
associated with INS resistance, aging, and increased proliferative activity of normal and neoplastic tissues. The dissociation constants (Kd) of human INS to human INSR and
IGF-1R are 0.23 and 16nM, respectively. IGF-I binds to
hybrid receptors with at least a 50-fold higher affinity than
INS, irrespective of the splicing variant. Guinea pig INSR
shows an exceptionally higher affinity (Kd= 0.083nM).
Several fish species have multiple INSR genes that show
different expression patterns among tissues. Human
INS binds to fish INSR at a Kd of 0.67 and 0.15nM in the
trout and carp, respectively. | [Agonists and Antagonists]
IGFs bind to INSR, but INS binds to IGF-IR with low
affinity. Several synthetic peptides (S519 and RB539)
show agonistic effects with a high affinity to INSR. Fish
INSs bind mammalian INSR with high affinity and vice
versa. RB537, S661, and S961 are INSR antagonists with high
affinity. S961 shows an agonistic effect only on 3H-thymidine incorporation | [Biological functions]
INS is a key regulator of glucose homeostasis in mammals because the peptide is the only hormone that lowers
blood glucose levels. INSR-A mainly enhances the
effects of IGF-II during embryogenesis and fetal development, whereas INSR-B is predominantly expressed in
adults and enhances the metabolic effects of INS. ProINS binds to INSR-A, which is present in the nervous
system, to elicit antiapoptotic and neuroprotective effects
in the developing and postnatal nervous system. In the
pancreas, the exocrine and endocrine components stimulate each other via intrapancreatic axes of communication. The insular-acinar axis is involved in the
regulation of pancreatic digestive enzyme production
in acinar cells located in the blood downstream of β-cells,
and the acini-insular axis is involved in the regulation of
INS release by pancreatic enzymes.
 | [Clinical implications]
Diabetes is classified as type 1 or type 2. Type 1 diabetes (INS-dependent diabetes mellitus) results from the
autoimmune destruction of β-cells. Type 2 diabetes
(noninsulin-dependent diabetes mellitus) is a metabolic
disorder characterized by hyperglycemia due to an absolute or relative lack of INS or cellular resistance to INS. In
mammals and fish, the administration of alloxan or streptozotocin selectively destroys β-cells, producing a type 2
diabetes model. |
| Hazard Information | Back Directory | [Chemical Properties]
White or almost white powder. | [Uses]
Antidiabetic. | [Brand name]
Humulin (Lilly);
Novolin (Novo Nordisk); Velosulin (Novo Nordisk). | [Description]
Insulin (INS) is the only hormone lowering blood glucose
levels in vertebrates. However, the importance of insulin in
the carbohydrate metabolism may differ between mammals
and other vertebrates. Frederick Banting and Charles Best discovered a substance that lowered blood sugar levels in the dog pancreas in 1921, and this was immediately applied to
diabetes care. The primary structure of INS has been
reported in many vertebrates, including agnathans, fish,
and tetrapods. | [General Description]
The INS gene encodes for preproinsulin, which is enzymatically converted into insulin. Insulin is produced in the insulin-producing pancreatic β cells. Preproinsulin is converted to proinsulin in ER and proinsulin is then proteolytically processed to form insulin in newly-forming insulin secretory granules. Insulin production is tightly regulated by specific DNA elements present within ~400 bp in the proximal region of the INS promoter. | [Biochem/physiol Actions]
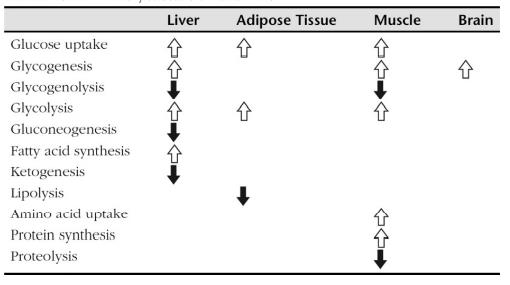
Insulin is responsible for two types of actions- excitatory and inhibitory. In its excitatory role, it increases the uptake of glucose and lipid synthesis, and in its inhibitory role it inhibits glycogenolysis, gluconeogenesis, lipolysis, proteolysis and ketogenesis. Aberrant insulin secretion leads to various disorders such as diabetes, hyperglycemia or hypoglycaemia. Type I diabetes is a result of autoimmune destruction of β cells of pancreas, which leads to depletion of insulin. Mutant INS-gene Induced Diabetes of Youth (MIDY) syndrome is an autosomal dominant disorder caused by missense mutations, which lead to aberrant proinsulin folding. Impaired glucose tolerance (IGT) or non-insulin-dependent diabetes mellitus (NIDDM) is caused by resistance to insulin-stimulated glucose uptake. | [Clinical Use]
In mammals and chickens, INS levels are routinely
measured using commercial kits, and several studies
have established specific immunoassays for fish. Many
INS analogs, which show different durations of action,
have been developed to treat diabetes. Alternative INS
administration methods other than injection are via the
lungs, nose, skin, and mouth. These have been developed using the insulin encapsulation technique with
micro- or nanodelivery systems. Many medical drugs
that stimulate INS release, both directly and indirectly,
are commercially available. Sulfonylurea and glinides
stimulate β-cells directly. The incretin GLP-1 analog can
lower glucose concentrations by augmenting insulin
secretion and suppressing glucagon release. In addition the dipeptidyl peptidase-4 (DPP-4) inhibitor inhibits
incretin degradation and indirectly enhances the stimulation of incretin to β-cells. Thiazolidinedione improves the
action of INS in the muscle and adipose tissue. Cone snail
(Conus geographus) G1 is a naturally occurring B-chainminimized mimetic of INS, which strongly binds to
human and fish INSR and activates receptor signaling.
This peptide induces extremely rapid hypoglycemic
shock. | [in vitro]
methotrexate (mtx) was found to be linked to insulin covalently. as effectively as insulin, insulin-mtx complex competed with 125i-insulin for insulin receptor. it was found that ic50 and ki for insulin-mtx were 93.82 nm and 91.88 nm, respectively, whereas those for insulin were 5.01 nm and 4.85 nm, respectively [1]. | [in vivo]
previous study showed that insulin-stimulated glucose uptake in extensor muscles from sjl mice was reduced, but the basal uptake rates were not different. in another mouse study, knockdown of tc10α but not tc10β in 3t3-l1 adipocytes resulted in a inhibition of both insulin-stimulated glucose uptake and glut4 translocation [2]. | [IC 50]
5.01 nmol/l for insulin receptor | [storage]
Store at -20°C | [Structure and conformation]
Human preproINS consists of 110 aa residues: 24 in the
signal peptide, 30 in the B-chain, 31 in the C-peptide, and
21 in the A-chain. The signal peptide and
C-peptide are excised from the preproINS to produce
the mature peptide, consisting of heterodimeric A- and
B-chains. Six cysteine residues are conserved throughout evolution from agnathans to mammals. Furthermore, the N-terminal seven aa residues of the A-chain
and the receptor binding region of the B-chain (Gly-Phe-Phe-Tyr) are highly conserved. Mr 5808, pI 5.3. INS is soluble under acidic and alkaline
conditions and almost insoluble at neutral pH. INS can be
stored for up to 6 months at 4°C in 1M acetic acid. Long-term
storage at an alkaline pH increases the rate of deamidation
and aggregation. One IU is equivalent to 0.0347mg
human INS.
 | [References]
[1] ou x,kuang a,liang z,peng x,zhong y. the binding characteristics of insulin-mtx to insulin receptor. hua xi yi ke da xue xue bao.2001 dec;32(4):538-40.
[2] leney se,tavaré jm. the molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. j endocrinol.2009 oct;203(1):1-18. |
|
|