| Identification | More | [Name]
Lithium oxide | [CAS]
12057-24-8 | [Synonyms]
dilithium monoxide
LITHIUM OXIDE
dilithiumoxide
Li2O
Lithium oxide (Li2O)
lithiummonoxide
Lithiumoxid
lithiumoxide(li2o)
oxydedelithium
Lithiumoxideminwhitepowder
LITHIUM OXIDE 98%MIN
LITHIUM OXIDE, POWDER,-60 MESH, 97%
LITHIUM OXIDE,-6MM +100 MESH
Lithia
Lithiumoxide,min.95%(99.5%-Li)
LITHIUM OXIDE (99.5%-LI)
Lithium oxide, 99.5% (metals basis)
lithium oxide fume
Oxybislithium
Oxydilithium | [EINECS(EC#)]
235-019-5 | [Molecular Formula]
Li2O | [MDL Number]
MFCD00016183 | [Molecular Weight]
29.88 | [MOL File]
12057-24-8.mol |
| Hazard Information | Back Directory | [Chemical Properties]
finely divided white powder(s) or crusty material; readily absorbs CO2 and H2O from the atmospheric; made by heating LiOH to ~800°C in a vacuum or by thermal decomposition of lithium peroxide; used in ceramics and special glass formulations and in lithium thermal batteries [HAW93] [MER06] [KIR81] [FMC93] | [Uses]
Ceramics and special glass formulations, carbon dioxide absorbent | [General Description]
Lithium oxide is a white crystalline solid. Its a strong base. It reacts with water and forms lithium hydroxide. | [Structure and conformation]
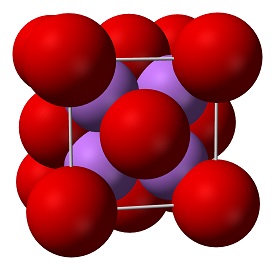
Solid lithium oxide adopts an antifluorite structure with four-coordinated Li+ centers and eight-coordinated oxides.The ground state gas phase Li2O molecule is linear with a bond length consistent with strong ionic bonding.VSEPR theory would predict a bent shape similar to H2O.
|
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 3262 8/PG 2
| [WGK Germany ]
3
| [RTECS ]
OJ6360000
| [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
II |
| Questions And Answer | Back Directory | [Chemical Properties]
Lithium oxide (Li2O) is one of simplest ionic oxides and it is isoelectronic to H2O. Two lithium atoms will each give one electron to the oxygen atom. forms the ionic bond between lithium and oxygen. The formula for lithium oxide is Li2O.
Lithium oxide is very corrosive. It reacts with water to make lithium hydroxide. It is toxic because of its strong alkalinity (being a base).
It is a highly insoluble thermally stable Lithium source suitable for glass, optic and ceramic applications. Lithium oxide is a white solid also known as lithia, it is produced when lithium metal burns in the presence of oxygen. Oxide compounds are not conductive to electricity. However, certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systems. They are compounds containing at least one oxygen anion and one metallic cation.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them. Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating.
| [Uses]
There are no current industrial uses which consume large quantities of lithium oxide.
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them.Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating.
| [Reactions]
Lithium oxide reacts with water as it dissolves to form a solution of lithium hydroxide.
Lithium oxide is a strong base and reacts typically with acidic gases and liquids to form lithium salts. At elevated temperatures, lithium oxide also reacts with many solid nonmetal oxides (SiO2, B2O3, etc.) and metal oxides (A12O3, Fe2O3, etc.). High-temperature reactions are the basis for the fluxing action of lithium oxide, lithium hydroxide and lithium carbonate. Care must be taken to avoid the reaction of lithium oxide with reaction vessels at high temperatures.
| [Preparation]
Lithium oxide is prepared by heating lithium metal in dry oxygen above 100°C:
4Li + O2→2Li2O
Another method of preparation that yields pure lithium oxide involves thermal decomposition of lithium peroxide:
2Li2O2→2Li2O + O2
Also, the oxide can be produced by heating the pure lithium hydroxide at 800°C in a vacuum:
2LiOH→Li2O + H2O
| [Health Hazard]
To the best of our knowledge the chemical, physical and toxicological properties of lithium oxide have not been thoroughly investigated and reported.
The toxicity of lithium compounds is a function of their solubility in water. Lithium ion has central nervous system toxicity. The initial effects of lithium exposure are tremors of the hands, nausea, micturition, slurred speech, sluggishness, sleepiness, vertigo, thirst, and increased urine volume. Effects from continued exposure are apathy, anorexia, fatigue, lethargy, muscular weakness, and changes in ecg. Long-term exposure leads to hypothyroidism, leukocytosis, edema, weight gain, polydipsia/polyuria (increased water intake leading to increased urinary output), memory impairment, seizures, kidney damage, shock, hypotension, cardiac arrhythmias, coma, death.
|
|
|