| Identification | More | [Name]
Sulfamerazine | [CAS]
127-79-7 | [Synonyms]
2-SULFANILAMIDO-4-METHYLPYRIMIDINE
4-AMINO-N-[4-METHYL-2-PYRIMIDINYL]-BENZENESULFONAMIDE
4-AMINO-N-(4-METHYL-PYRIMIDIN-2-YL)-BENZENESULFONAMIDE
4-METHYL-2-SULFANILAMIDO-PYRIMIDINE
DEBENAL
LABOTEST-BB LT00455132
METHYLPYRIMAL
METSULFA
N1-(4-METHYLPYRIMIDIN-2-YL)SULFANILAMIDE
PYRIMAL-M
SULFAMERAZINE
SULFAMETHYLDIAZINE
(p-Aminobenzolsulfonyl)-2-amino-4-methylpyrimidin
2-(4-Aminobenzenesulfonamido)-4-methylpyrimidine
2-(p-Aminobenzolsulfonamido)-4-methylpyrimidine
2632 R. P.
2643-RP
2-Sulfa-4-methylpyrimidine
4-amino-n-(4-methyl-2-pyrimidinyl)-benzenesulfonamid
A-310 | [EINECS(EC#)]
204-866-2 | [Molecular Formula]
C11H12N4O2S | [MDL Number]
MFCD00023212 | [Molecular Weight]
264.3 | [MOL File]
127-79-7.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
2
| [RTECS ]
WP0750000
| [F ]
10 | [TSCA ]
Yes | [HS Code ]
2935904000 | [Safety Profile]
Moderately toxic by intravenous and subcutaneous routes. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NO, and SOx,. | [Toxicity]
LD50 oral in mouse: 25gm/kg |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Sulfamerazine,Lederle,US,1943 | [Uses]
Antibacterial. | [Definition]
ChEBI: A sulfonamide consisting of pyrimidine with a methyl substituent at the 4-position and a 4-aminobenzenesulfonamido group at the 2-position. | [Manufacturing Process]
To a well agitated solution of 6.95 grams of 2-amino-6-methyl pyrimidine in
40 cc of pyridine, 15 grams of p-acetylaminobenzenesulfonyl chloride are
added in small portions over a 30 minute period. The reaction mixture is then
heated on a steam bath for 30 minutes, the free pyridine being then removed
under reduced pressure and the residue mixed with cold water, and the latter
mixture is vigorously stirred. The solid reaction product is removed by
filtration and washed with cold water.
There is obtained a yield of 14 grams of crude 2-(pacetylaminobenzenesulfonamido)-
6-methyl pyrimidine, which on
recrystallization from alcohol and water melts at 238° to 239°C. The crude
product is hydrolyzed by suspending it in 400 cc of 2 N hydrochloric acid and
warming until solution is complete. The solution is neutralized with sodium
carbonate and the precipitated 2-(sulfanilamido)-6-methyl pyrimidine is
removed by filtration. The latter on recrystallization from alcohol and water
shows a melting point of 225° to 226°C. | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
Like all examined sulfanilamides, this drug is effective in treating infections caused by
streptococci, gonococci, pneumococci, staphylococci, and also colon bacillus. Synonyms
of this drug are dosulfin, polagin, romezin, and others. | [General Description]
Chemical structure: sulfonamide | [Pharmaceutical Applications]
2-Sulfonamido-4-methylpyrimidine. A component of some
triple sulfa combinations. Plasma half-life is c. 24 h and protein
binding c. 75%. It is less active than sulfadiazine. | [Synthesis]
Sulfamerazine, N1
-(4-methyl-2-pyrimidinyl)sulfanilamide (33.1.12), is
also synthesized in the manner described above, which is by reacting 4-acetylaminoben�zenesulfonyl chloride with 2-amino-4-methylpyrimidine (33.1.10), which is in turn syn�thesized by the traditional scheme of synthesizing derivatives of pyrimidine. Acetoacetic
ester is condensed with guanidine to give 4-methyl-2-aminopyrimidin-6-one (33.1.8).
Reacting this with phosphorous oxychloride gives 4-methyl-2-amino-6-chloropyrimidine
(33.1.9). The chlorine atom at C6 of the pyrimidine ring is then removed by reduction with
hydrogen using a palladium on carbon catalyst. The resulting 4-methyl-2-aminopyrimi�dine (33.1.10) is then reacted with 4-acetylaminobenzenesulfonyl chloride to make an
acetanilide derivative (33.1.11), the subsequent hydrolysis of which with base leads to the
formation of the desired sulfamerazine.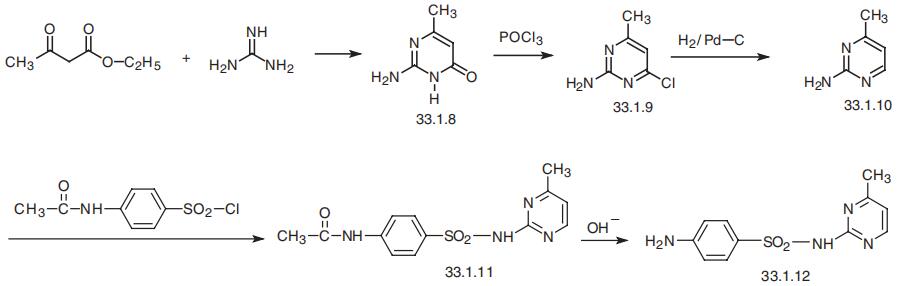
| [storage]
Store at -20°C |
|
|