| Identification | More | [Name]
DL-10-CAMPHORSULFONIC ACID | [CAS]
5872-08-2 | [Synonyms]
(+/-)-10-CAMPHORSULFONIC ACID
10-CAMPHORSULFONIC ACID
(+/-)-CAMPHOR-10-SULFONIC ACID
CAMPHOR-10-SULFONIC ACID
(+/-)-CAMPHOR-10-SULFONIC ACID (BETA)
(+/-)-CAMPHOR-10-SULPHONIC ACID
D-(+)-10-CAMPHORSULFONIC ACID MONOHYDRATE
DL-10-CAMPHORSULFONIC ACID
DL-10-CAMPHORSULFONIC ACID N-HYDRATE
DL-10-CAMPHORSULPHONIC ACID
DL-CAMPHOR-10-SULFONIC ACID
DL-CAMPHORSULFONIC ACID
DL-OXO-10-BORNANESULFONIC ACID
7-dimethyl-2-oxo-(+/-)-bicyclo[2.2.1]heptane-1-methanesulfonicaci
DL-6-oxobornane-10-sulphonic acid
D,L-Camphor sulphonic acid
(±)-Camphor-10-sulfonicacid,98%
Bicyclo2.2.1heptane-1-methanesulfonic acid, 7,7-dimethyl-2-oxo-
(n)-camphor-10-sulfonic acid
Campho 10 Sulphonic Acid | [EINECS(EC#)]
227-527-0 | [Molecular Formula]
C10H16O4S | [MDL Number]
MFCD00074827 | [Molecular Weight]
232.3 | [MOL File]
5872-08-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
C | [Risk Statements ]
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S27:Take off immediately all contaminated clothing . | [RIDADR ]
UN 3261 8/PG 2
| [WGK Germany ]
3
| [RTECS ]
DT5077100 | [F ]
3-10 | [TSCA ]
Yes | [HazardClass ]
8 | [PackingGroup ]
III | [HS Code ]
29147000 |
| Questions And Answer | Back Directory | [Overview]
DL-Camphorsulfonic acid, also known as camphorsulfonic acid, is a white or almost white crystalline powder. The molecular formula of camphorsulfonic acid is C10H16O4S. It is a pair of chiral optical enantiomers with prismatic crystals, divided into left-handed camphorsulfonic acid and right-handed camphorsulfonic acid. The melting point is 193~195 ℃ and 197~200 ℃, respectively. Camphorsulfonic acid is a derivative of camphor, widely used in medicine, light industry and cosmetic industry. At present, its application mainly focused on chiral asymmetric synthesis, high selective catalysis of natural products and polymer doping with polyaniline to prepare nano-scale conductive polymer materials.
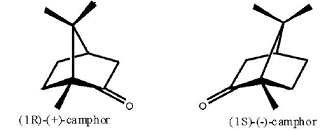
Figure 1: left-right isomer of camphorsulfonic acid | [Preparation]
natural camphor is mainly extracted from camphor plants, ginger plants and sage seeds, and with optical activity; synthetic camphor is generally prepared by the isomerization of turpentine to obtain camphene, then by esterification, hydrolysis and dehydrogenation reaction process; synthetic camphor is racemic, almost lacking optical activity. Through the reaction of camphor powder and concentrated sulfuric acid sulfonation, racemic camphorsulfonic acid is obtained, and then by the resolution, left and right handed camphorsulfonic acid is made. The optimum conditions for the synthesis of camphorsulfonic acid are as follows: the molar ratio of camphor powder, concentrated sulfuric acid and acetic anhydride is 1.0: 1.4: 3.0; reaction temperature is10 ℃; standing time is 5 days, and camphorsulfonic acid is vacuum dried. Utilizing sopropyl alcohol as solvent, camphor sulfonate is obtained by neutralization of camphorsulfonic acid and a resolving agent made by alcohol amine; camphorsulfonic acid is obtained by purification.
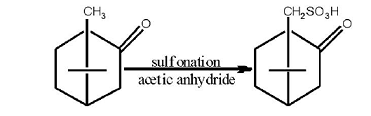
Figure 2: Preparation principle | [Resolution]
Kinetic resolution is the selective chiral reduction of racemic camphor, so that one of the enantiomers of racemates is selectively reducted, leaving the required isomers, and then purifying. It is also possible to add optically active amino acids, phenylglycine, phenylalanine, hydroxyphenylglycine, o-chlorophenylglycine and resolving agents of its derivatives. Subjecting racemic camphorsulfonic acid to salting treatment, separating the left and right camphorsulfonic acid ammonium salt solution by chromatography, film evaporation, crystallization and filtration; drying to get white crystals, the left and right handed camphorsulfonic acid is made. | [Uses]
Pharmaceutical intermediates, optical split agent. Camphorsulfonic acid is an important organic synthesis intermediates, optical resolving agent. Due to its optical rotation, it is industrially applicable be the optical isomers for racemisation, and is also widely used as pharmaceutical intermediates such as for the production of intestinal disorder inhibitors, HIV improving agents and the like. Left and right handed camphorsulfonic acid is an important resolving agent for optically active amino acids, such as camphorsulfonic acid—a chiral ion-pairing reagent. It separates 5 substances—phenylpropanolamine, which has receptor-blocking action and cardiac inhibition and local anesthetic action effects; metoprolol, propranolol, epinephrine, salbutamol and atenolol.
Camphor sulfonate made by camphorsulfonic acid for salting or other synthetic routes also has a wide range of uses. For example, camphor sulfonate is a veterinary central stimulant, which can stimulate the respiratory center and cause respiratory excitement, ; it is used for the treatment respiratory and circulatory acute disorders, resisting central nervous system poisoning; camphor ammonium sulfonate acts as a chiral ion pair reagent added to the mobile phase to separate the aromatic alcohol amine drug enantiomers.

Figure 3: Camphor sulfonate | [Market]
The domestic demand for camphorsulfonic acid is high; camphorsulfonic acid is basically dependent on imports, and the price of it is also very expensive, especially for L-camphorsulfonic acid. | [References]
[1] KE Chunlan, LIU Xiaohong, XU Chunxiao etc. Synthesis and Resolution of Camphor Sulfonic Acid [J]. Journal of Nanchang University Engineering, 2010, 32 (4): 381-384.
[2] KE Chunlan, LIU Xiaohong, XU Chunxiao etc. Synthesis and latest application of chiral camphorsulfonic acid and its salts [J] .Jiangxi Chemical Industry, 2010 (2): 1-4. |
| Hazard Information | Back Directory | [Chemical Properties]
DL-10-CAMPHORSULFONIC ACID is white to off-white powder
| [Definition]
ChEBI: Camphorsulfonic acid is a sulfonic acid containing the camphorsulfonate anion. It is functionally related to a camphor. It is a conjugate acid of a camphorsulfonate anion. | [General Description]
Camphorsulfonic acid is a organosulphur compound. |
|
|