| Identification | More | [Name]
6-Mercaptopurine monohydrate | [CAS]
6112-76-1 | [Synonyms]
6-MERCAPTOPURINE HYDRATE
6-MERCAPTOPURINE MONOHYDRATE
6MP H2O
6MP MONOHYDRATE
6-PURINETHIOL MONOHYDRATE
6-thiohypoxanthine monohydrate
7H-PURINE-6-THIOL HYDRATE
LEUKERIN MONOHYDRATE
MERCALEUKIN MONOHYDRATE
MERCAPTOPURINE
MERCAPTOPURINE, MONOHYDRATE
MERCAPTOPURINE MONOHYDRATE, 6-
PURINE-6-THIOL, MONOHYDRATE
1,7-dihydro-6h-purin-6-thion,monohydrat
1,7-dihydro-6h-purine-6-thionemonohydrate
1,7-dihydro-6h-purine-6-thionmonohydrate
6h-purin-6-thion,monohydrat
6-merkaptopurin,monohydrat
purin-6-thiol,monohydrat
6-MERCAPTOPURIN HYDRAT 99% | [EINECS(EC#)]
212-968-3 | [Molecular Formula]
C5H6N4OS | [MDL Number]
MFCD03854445 | [Molecular Weight]
170.19 | [MOL File]
6112-76-1.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal powder | [Melting point ]
>300 °C(lit.) | [storage temp. ]
Store at 0-5 | [solubility ]
INSOLUBLE | [form ]
Fine Powder | [color ]
Yellow | [Stability:]
Stable. Incompatible with strong oxidizing agents, acids, strong bases. Light sensitive. | [Water Solubility ]
INSOLUBLE | [Usage]
An immunosuppressive drug used to treat leukemia. It is also used for pediatric non-Hodgkin lymphoma, polycythemia vera, and psoriatic arthritis | [Merck ]
14,5871 | [BRN ]
4012091 | [BCS Class]
4 | [CAS DataBase Reference]
6112-76-1(CAS DataBase Reference) | [EPA Substance Registry System]
6112-76-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R63:Possible risk of harm to the unborn child.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
UN 2811 6.1 / PGIII | [WGK Germany ]
3
| [RTECS ]
UP0400000
| [HS Code ]
29335995 |
| Hazard Information | Back Directory | [General Description]
Odorless light yellow to yellow crystalline powder. Becomes anhydrous at 284°F. | [Reactivity Profile]
6-MERCAPTOPURINE MONOHYDRATE(6112-76-1) reacts with strong oxidizing agents, strong bases and strong acids. | [Air & Water Reactions]
This compound is sensitive to light and oxidation. Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available. 6-MERCAPTOPURINE MONOHYDRATE is probably combustible. | [Description]
6-Mercaptopurine (6-MP) is an inhibitor of purine synthesis and interconversion.1 It is rapidly converted to 6-mercaptopurine ribonucleoside-5''-monophosphate, which inhibits phosphoribosyl pyrophosphate (PRPP) amidotransferase, the rate-limiting enzyme in purine synthesis. It also inhibits the conversion of IMP to adenylosuccinic acid and xanthylic acid and blocks AMP formation in vitro. 6-MP (30 mg/kg) inhibits growth of sarcoma 180, adenocarcinoma E 0771, and adenocarcinoma 755 tumors and reduces the size of leukemia L1210 subcutaneous growths in mice.2 It also decreases delayed-type hypersensitivity and thyroid inflammation in a guinea pig model of thyroiditis when administered pre- or post-disease onset.3 Formulations containing mercaptopurine have been used for maintenance therapy in patients with acute lymphoblastic leukemia.4 | [Chemical Properties]
white to light yellow crystal powder | [Uses]
An immunosuppressive drug used to treat leukemia. It is also used for pediatric non-Hodgkin lymphoma, polycythemia vera, and psoriatic arthritis | [Uses]
An immunosuppressive drug used to treat leukemia. It is also used for pediatric non-Hodgkin’s lymphoma, polycythemia vera, and psoriatic arthritis. | [Uses]
antihypertensive, ACE inhibitor | [Definition]
ChEBI: Mercaptopurine hydrate is a hydrate. It contains a mercaptopurine. | [Indications]
Mercaptopurine (Purinethol) is an analogue of hypoxanthine
and was one of the first agents shown to be active
against acute leukemias. It is now used as part of
maintenance therapy in acute lymphoblastic leukemia.
Mercaptopurine must be activated to a nucleotide by
the enzyme HGPRTase. This metabolite is capable of
inhibiting the synthesis of the normal purines adenine
and guanine at the initial aminotransferase step and inhibiting
the conversion of inosinic acid to the nucleotides
adenylate and guanylate at several steps.
Some mercaptopurine is also incorporated into DNA in
the form of thioguanine. The relative significance of
these mechanisms to the antitumor action of mercaptopurine
is not clear.
Resistance to mercaptopurine may be a result of decreased
drug activation by HGPRTase or increased inactivation
by alkaline phosphatase.
The plasma half-life of an intravenous bolus injection
of mercaptopurine is 21 minutes in children and 47
minutes in adults. After oral administration, peak
plasma levels are attained within 2 hours. The drug is
20% bound to plasma proteins and does not enter the
CSF. Xanthine oxidase is the primary enzyme involved
in the metabolic inactivation of mercaptopurine.
Mercaptopurine is used in the maintenance therapy
of acute lymphoblastic leukemia. It also displays activity
against acute and chronic myelogenous leukemias.
The major toxicities of mercaptopurine are myelosuppression,
nausea, vomiting, and hepatic toxicity. | [Brand name]
Purinethol (Teva). | [Mechanism of action]
Because the major mechanism of action of mercaptopurine is inhibition of de novo purine nucleotide biosynthesis rather than apoptosis secondary to the incorporation of false nucleotides into DNA, there is a lower risk for mutagenesis and secondary malignancy compared to thioguanine. | [Clinical Use]
Mercaptopurine is used in the treatment of acute lymphatic and myelogenous leukemias. | [Side effects]
Bone marrow suppression is the major use-limiting toxicity, although the drug can be hepatotoxic in high doses. Dosage adjustments should be considered in the face of renal or hepatic impairment. | [Synthesis]
Mercaptopurine, 6-purinthiol, is made from uric acid (30.1.2.5), which is
synthesized from barbituric acid (30.1.2.1). Barbituric acid (30.1.2.1) is easily made
by condensing urea with malonic ester and then nitrosylating it with nitrous acid. The
nitrosoderivative (30.1.2.2) is reduced by hydrogen (obtained in situ by reacting tin with
hydrochloric acid) to an amine (uramil) (30.1.2.3), and then reacted with isocyanic acid,
which forms pseudouric acid (30.1.2.4). This undergoes cyclization to uric acid (30.1.2.5)
when heated in the presence of hydrochloric acid. Upon reacting phosphorous pentachlo�ride with uric acid, 2,6,8-trichloropurine (30.1.2.6) is formed. The three chlorine atoms in
trichloropurine differ significantly in terms of reactivity for nucleophilic substitution. The
chlorine atom at C6 is much more active than the chlorine atom at C2, and this is more active
than the chlorine atom at C8, which allows subsequent manipulation by them. Interaction of
2,6,8-trichloropurine (30.1.2.6) with sodium hydroxide allows to replace the chlorine atom
at C6, forming the dichloro-derivative (30.1.2.7), which is then reduced by hydriodic acid to
hypoxanthine (30.1.2.8). Upon reaction with phosphorous pentasulfide, hypoxanthine is
transformed into mercaptopurine (30.1.2.9).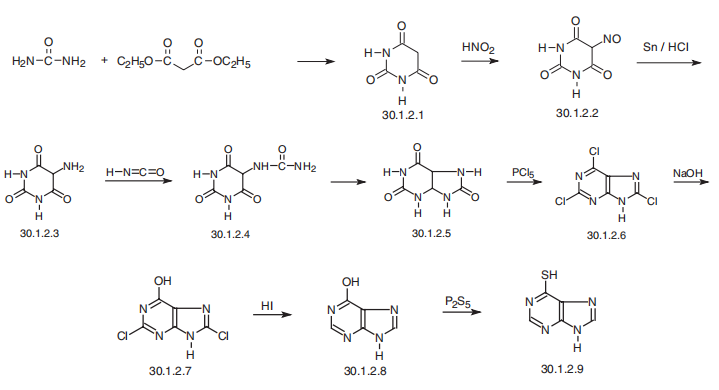
| [Drug interactions]
Potentially hazardous interactions with other drugs
Allopurinol: decreased rate of metabolism of
mercaptopurine - reduce dose of mercaptopurine to
a quarter of normal dose.
Antibacterials: increased risk of haematological
toxicity with co-trimoxazole and trimethoprim.
Anticoagulants: possibly reduced anticoagulant effect
of coumarins.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Febuxostat: avoid concomitant use. | [Metabolism]
It is available in an oral dosage form, but absorption can be erratic and is reduced by the presence of food. The drug is extensively metabolized on first pass and excreted by the kidneys. | [Purification Methods]
Crystallise 6-mercaptopurine from pyridine (30mL/g), wash it with pyridine, then triturate with water (25mL/g) and adjust to pH 5 by adding M HCl. Recrystallise it by heating, then cooling, the solution. Filter off the solid, wash it with water and dry it at 110o. It has also been crystallised from water (charcoal) as yellow crystals of the monohydrate which become anhydrous on drying at 140o. It has UV: at 230 and 312nm ( 14,000 and 19,600) in 0.1N NaOH; 222 and 327nm ( 9,2400 max and 21,300), and 216 and 329nm ( 8,740 and 19,300) in MeOH. It forms a 1:1 complex with Zn2+ , Pb2+ , Co2+, and Ni2+ in aqueous dioxan. It is an antineoplastic. [Albert & Brown J Chem Soc 2060 1954, IR: Brown & Mason J Chem Soc 682 1957, UV: Fox et al. J Am Chem Soc 80 1669 1958, UV: Mason J Chem Soc 2071 1954, Beilstein 26 III/IV 2097.] |
|
|