| Identification | Back Directory | [Name]
Kallikrein | [CAS]
9001-01-8 | [Synonyms]
2HK
sk-827
padutin
kalleone
glumorin
onokreinp
padreatin
dilminald
bradykini
callicrein
KALLIKREIN
KININOGENIN
e.c.3.4.21.8
e.c.3.4.4.21
EC 3.4.21.35
EC 3.4.21.34
KININOGENASE
Dpot-padutin
urokallikrein
depot-padutin
LMW kininogen
kallidinogenase
HUMAN KALLIKREIN
KALLIKREIN, HUMAN
urinarykallikrein
tissue kallikrein
Plasma:Kallikrein
pancreatickallikrein
KALLIKREIN, HUMAN URINE
Pancreatic Kininogenase
Kallikrein,Plasma/CH8530
KALLIKREIN, HUMAN PLASMA
Kininogenase, Kininogenin
Human urine kallidinogenase
Urine kallidinogenase【human】
KALLIKREIN, PORCINE PANCREAS
kallikrein from human plasma
Kallikreinfromplasma,poricine
KININOGEN, LOW MOLECULAR WEIGHT
kallikrein from porcine pancreas
KALLIKREIN FROM PORCRINE PANCREAS
Kallikerin,from Porcine Pancreas
KALLIKREIN, PORCINE PANCREAS TISSUE
KININOGEN, HUMAN, HIGH MW, DOUBLE CHAIN
KALLIKREIN(EC 3.4.21.35) PORCINE PANCREAS
KININOGEN HIGH MOLECULAR WEIGHT, TWO CHAIN
KININOGEN, HIGH MOLECULAR WEIGHT, TWO CHAIN, HUMAN
Kininogen, low molecular weight from human plasma
KININOGEN, HIGH MOLECULAR WEIGHT, TWO CHAIN, HUMAN PLASMA
Kininogen high molecular weight, Two Chain from human plasma
| [EINECS(EC#)]
232-574-5 | [MDL Number]
MFCD00131428 |
| Questions And Answer | Back Directory | [Structure]
Plasma kallikrein possesses a unique structure in vertebrates and is composed of four apple domains followed
by a trypsin domain. The apple domain is a
conserved protein folding with three disulfide bridges,
and is often found on diverse proteins for protein-protein
or protein-carbohydrate interactions. The apple domains
on KLKB1 interact with the D6 domain of molecular
weight kininogen (HMW-KNG), which is highly glycosylated. In teleost genomes, the KLKB1-like gene is absent
and only lectins with four apple domains but no trypsin
domain were found. These lectins were not found in
other vertebrates, suggesting that the two genes could
share the same origin but the trypsin domain was lost
in the teleost lineage during evolution. Tissue kallikreins
possess a single trypsin domain and are closely related to
other serine proteases. It is not clear what enzyme is
involved in the cleavage of KNG to form bradykinin
(BK) in the fish and lamprey, given that KLKB1 and
KLK are not identified.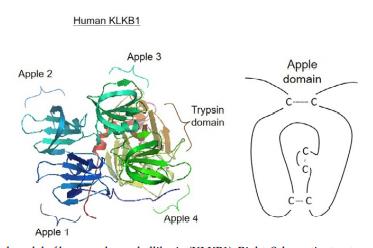 | [Gene and mRNA]
Plasma kallikrein (EC 3.4.21.34) is transcribed by
KLKB1 located on chromosome 4q35 in humans. In mammals, KLKB1 is duplicated to form factor XI (F11) by tandem duplication.1 KLKB1 in plasma is mostly in an
inactive form known as prekallikrein, and it is activated
by contact activation involving HMW-KNG and factor
XII. Tissue kallikreins (EC 3.4.21.35) are transcribed by
the KLK genes located on chromosome 19q13 in humans.
In tetrapods, the tissue kallikreins are tandemly duplicated into a large family (KLK1-KLK15 on human chromosome 19). Human KLKB1 possesses four apple
domains followed by a trypsin domain and KLK possesses only a trypsin domain. The sequences
of the trypsin domain are highly variable, but the catalytic domains (trypsin triads) are similar. KLK-like
sequences were identified in teleosts, but the catalytic
domains are mutated, indicating a possible loss of function of these enzymes and the genes became pseudogenes. Therefore, other trypsin-like enzymes may have
taken the role of these KLK-like enezymes in teleosts to
produce [Arg0
]-BK from KNG.
 | [Synthesis and release]
Although KLKB1 and F11 are tandem duplicated
orthologs, their regulations are different. Hepatic nuclear
factor 4α (HNF4α) deletion decreased F11 but not KLKB1.
Estrogen or thyroid hormone treatment increased F11
expression but not KLKB1 while a high-fat diet increased
both F11 and KLKB1 expression. For KLK, the regulation of synthesis and release was
most studied in detail in KLK3 and multiple androgenresponsive elements were identified upstream of KLK3. | [Biological functions]
KLKB1 selectively cleaves Arg/Xaa and Lys/Xaa
bonds, including Lys/Arg and Arg/Ser bonds in human
KNG, to release BK. It also digests plasminogen to plasmin, and participates in the surface-dependent activation
of blood coagulation, fibrinolysis, and inflammation. It
converts prorenin to renin to activate the RAS. Teleost
KLKB1-like lectin facilitates hemagglutination by binding to the pathogen-like glycoproteins, and could be
involved in immune function. KLK is highly selective to release [Lys0
]-BK from both
HMW- and LMW-KNGs, which involves the hydrolysis
of Met/Xaa or Leu/Xaa. Besides acting as an enzyme
for KKS, KLK is also involved in proteolytic cascades
for semen liquefaction through the hydrolysis of seminogelin and the desquamation of the skin by the cleavage of
cellular adhesion proteins. |
| Hazard Information | Back Directory | [Description]
Plasma kallikrein possesses a unique protein structure
with four apple domains and a trypsin domain, which evolved
before coagulation factor XI. Tissue kallikreins are trypsinbased enzymes, and some members are highly correlated with
prostate cancer. The evidence that human urine induces hypotension
when injected intravenously into anesthetized dogs was
first described in 1909. Two major kallikreins, plasma kallikrein (KLKB1) and tissue (glandular) kallikrein (KLK),
were found in mammals, and they were transcribed by
different genes. Glandular KLK was an old name and
was replaced by tissue KLK in the modern nomenclature. | [Uses]
Kallikrein from porcine pancreas has been used:
- as a matrix metalloproteinase-9 (MMP-9) zymogen activator
- as a component of cell culture to test its effect on rat subventricular zone (SVZ) cells and oligodendrocyte progenitor cells (OPC) proliferation and survival
- as a model enzyme to track kinetic data and visual detection limits of hydrolysis by hydrolytic enzymes in the two-phases array
| [General Description]
Kallikrein exists as an inactive prokallikrein in the porcine pancreas. The porcine kallikrein gene region is localized on chromosome 6q12-q21. | [Biochem/physiol Actions]
Kallikrein active forms are generated by the enzymatic action of trypsin. It is a serine protease that mediates the activation of growth factors and substrates. | [Clinical Use]
Kallikreins are drug targets for the control of hypertension, inflammation, and blood coagulation diseases. They
are also possible biomarkers for cancer. KLK2 and KLK3 [prostate-specific antigen (PSA)] are
used as the serum biomarker for prostate cancer. |
|
|