N-[(1S,5R)-7,9-dimethyl-7,9-diazabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide dihydrochloride Chemische Eigenschaften,Einsatz,Produktion Methoden
Originator
Nisshin (Japan)
Trademarks
Sinseron
Synthese
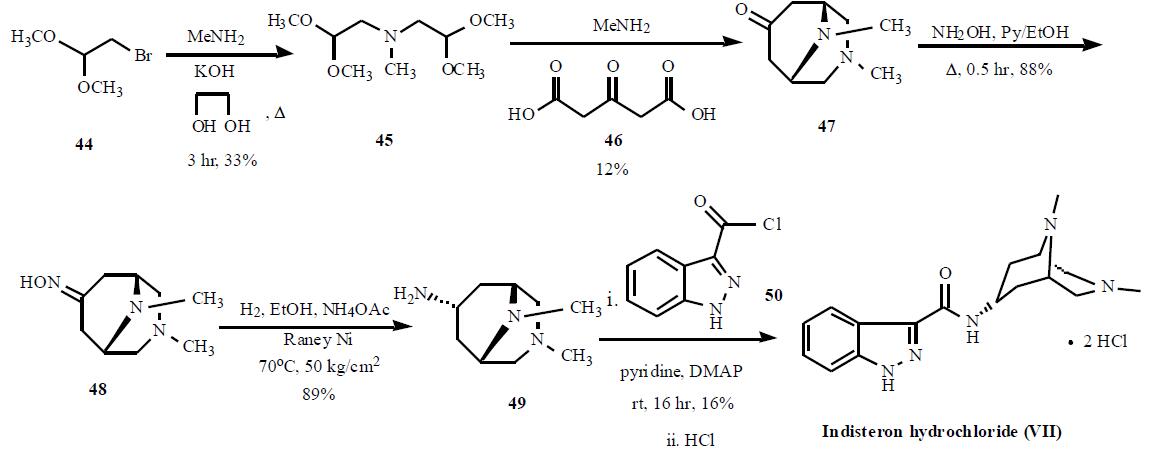
The synthesis is highlighted in the Scheme.
Bromoacetaldehyde dimethyl acetal (44) was condensed with
methylamine with KOH in refluxing ethyleneglycol for 3 hr
to give 33% yield of bis(2,2-dimethoxyethyl)amine (45),
which was cyclized with acetonedicarboxylic acid (46) and
methylamine to generate 3,9-dimethyl-3,9-diazabicyclo-
[3.3.1]nonan-7-one (47) in 12% yield. Compound 47 was
reacted with hydroxylamine in refluxing pyridine and ethanol
mixture to give corresponding oxime 48 in 88% yield,
which was subsequently reduced with hydrogen over Raney
Ni in hot ethanol in the presence of ammonium acetate at 50
kg/cm2 to give amine 49 in 89% yield. Compound 49 was condensed with 1H-indazole-3-carbonyl chloride (50) in
pyridine with catalytic amount of DMAP to give crude
indisteron free base, which was re-crystallized from
chloroform/hexane to give indisteron free base as colorless
crystals in 16% yield. Finally, the free base was treated with
hydrogen chloride to give indisteron hydrochloride (VII).
N-[(1S,5R)-7,9-dimethyl-7,9-diazabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide dihydrochloride Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

