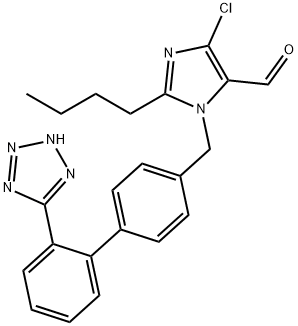
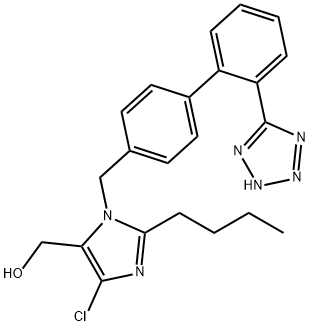
Losartan Carboxaldehyde
- CAS No.
- 114798-36-6
- Chemical Name:
- Losartan Carboxaldehyde
- Synonyms
- DUP 167;EXP 3179;Losartan Aldehyde;Losartan EP IMpurity K;Losartan Carboxaldehyde;Losartan Impurity K (EP);Losartan Carboxaldehyde-d3;Losartan Related CoMpound C;Losartan USP Related Compound C;Allisartan Isoproxil impurity II
- CBNumber:
- CB01176401
- Molecular Formula:
- C22H21ClN6O
- Molecular Weight:
- 420.89
- MDL Number:
- MFCD00870256
- MOL File:
- 114798-36-6.mol
| Melting point | 84-86°C |
|---|---|
| Boiling point | 666.7±65.0 °C(Predicted) |
| Density | 1.34±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator, Under Inert Atmosphere |
| solubility | Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.16±0.10(Predicted) |
| color | Off-White to Light Yellow |
| CAS DataBase Reference | 114798-36-6 |
| FDA UNII | KJ52CU0VV6 |
Losartan Carboxaldehyde price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 18855 | Losartan Carboxaldehyde ≥95% | 114798-36-6 | 1mg | $57 | 2024-03-01 | Buy |
| Cayman Chemical | 18855 | Losartan Carboxaldehyde ≥95% | 114798-36-6 | 5mg | $152 | 2024-03-01 | Buy |
| Cayman Chemical | 18855 | Losartan Carboxaldehyde ≥95% | 114798-36-6 | 10mg | $249 | 2024-03-01 | Buy |
| TRC | L470505 | LosartanCarboxaldehyde | 114798-36-6 | 50mg | $670 | 2021-12-16 | Buy |
| Usbiological | 281287 | Losartan carboxaldehyde | 114798-36-6 | 2mg | $345 | 2021-12-16 | Buy |
Losartan Carboxaldehyde Chemical Properties,Uses,Production
Description
Losartan carboxaldehyde is an intermediate aldehyde metabolite of the angiotensin II type 1 receptor antagonist, losartan . It does not block angiotensin receptors, but instead inhibits endothelial cyclooxygenase (COX)-2 expression, thereby exerting anti-inflammatory actions. At 1 μM in vitro, losartan carboxaldehyde has also been shown to block the upregulation of intercellular adhesion molecule (ICAM)-1 mRNA and COX-dependent generation of thromboxane A2 and prostaglandin F2α . Losartan carboxaldehyde can also act as a partial agonist (EC50 = 17.1 μM) of the insulin-sensitizing peroxisome proliferator-activated receptor γ in vitro.
Chemical Properties
Yellow Solid
Uses
A labelled intermediate in the synthesis of the EXP 3174, a metabolite of Losartan
Uses
A metabolite of Losartan. An intermediate in the synthesis of the EXP 3174
Uses
Losartan carboxaldehyde is an intermediate aldehyde metabolite of the angiotensin II type 1 receptor antagonist, losartan . It does not block angiotensin receptors, but instead inhibits endothelial cyclooxygenase (COX)-2 expression, thereby exerting anti-inflammatory actions. At 1 μM in vitro, losartan carboxaldehyde has also been shown to block the upregulation of intercellular adhesion molecule (ICAM)-1 mRNA and COX-dependent generation of thromboxane A2 and prostaglandin F2α . Losartan carboxaldehyde can also act as a partial agonist (EC50 = 17.1 μM) of the insulin-sensitizing peroxisome proliferator-activated receptor γ in vitro.[Cayman Chemical]
in vitro
losartan is an intermediate aldehyde metabolite of losartan, the angiotensin ii type 1 receptor antagonist. losartan could not block angiotensin receptors, but inhibit the expression of endothelial cyclooxygenase (cox)-2, therefore exerting anti-inflammatory actions. moreover, losartan at 1 μm was able to block the upregulation of icam-1 mrna and cox-dependent generation of thromboxane a2 and prostaglandin f2α [1].
in vivo
in animal stufdy, losartan was infused for 10 days to rats on a normal sodium intake (nna) and rats on a high sodium intake (hna) to suppress endogenous ang ii. although basal plasma renin activity was markedly suppressed in hna rats compared with nna rats, control arterial pressure was not different between nna and hna rats. losartan could decrease arterial pressure from control levels in nna rats on the first day of infusion but had no effect on arterial pressure in hna rats. in addition, by day 10 of losartan infusion, arterial pressure had decreased further from control levels in nna rats but remained unchanged compared with control in hna rats [2].
References
[1] c. kr mer, j. sunkomat, j. witte, et al. angiotensin ii receptor-independent antiinflammatory and antiaggregatory properties of losartan: role of the active metabolite exp3179. circulation research 90(7), 770-776 (2002).
[2] collister jp, hornfeldt bj, osborn jw. hypotensive response to losartan in normal rats. role of ang ii and the area postrema. hypertension. 1996 mar;27(3 pt 2):598-606.
[3] goa kl, wagstaff aj. losartan potassium: a review of its pharmacology, clinical efficacy and tolerability in the management of hypertension. drugs. 1996 may;51(5):820-45.
Losartan Carboxaldehyde Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12468 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| sgtlifesciences pvt ltd | +8617013299288 | dj@sgtlifesciences.com | China | 12382 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| Nanjing Doge Biomedical Technology Co., Ltd | +86-25-58227606 +86-15305155328 | sales@dogechemical.com | China | 4128 | 58 |
View Lastest Price from Losartan Carboxaldehyde manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
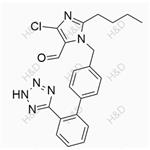 |
2024-04-02 | Allisartan Isoproxil Impurity 14
114798-36-6
|
US $0.00 / mg | 10mg | 0.98 | 10g | ShenZhen H&D Pharmaceutical Technology Co., LTD | |
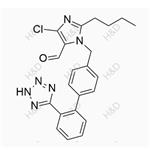 |
2024-04-02 | Allisartan Isoproxil Impurity 14
114798-36-6
|
US $0.00 / mg | 10mg | 0.98 | 10g | ShenZhen H&D Pharmaceutical Technology Co., LTD |
-

- Allisartan Isoproxil Impurity 14
114798-36-6
- US $0.00 / mg
- 0.98
- ShenZhen H&D Pharmaceutical Technology Co., LTD
-

- Allisartan Isoproxil Impurity 14
114798-36-6
- US $0.00 / mg
- 0.98
- ShenZhen H&D Pharmaceutical Technology Co., LTD
114798-36-6(Losartan Carboxaldehyde)Related Search:
1of4