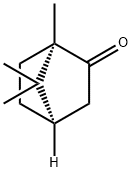
D-CAMPHOR
- CAS No.
- 464-49-3
- Chemical Name:
- D-CAMPHOR
- Synonyms
- Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1R)-;(1R,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one;d-camphre;FEMA 2230;d-2-Bornanone;7,7-trimethyl-(1r)-bicyclo(2.2.1)heptan-2-on;(1R)-1,7,7-TRIMETHYLBICYCLO[2.2.1]HEPTAN-2-ONE;D-CAMPHOR;camphorusp;Camphor usp
- CBNumber:
- CB0233316
- Molecular Formula:
- C10H16O
- Molecular Weight:
- 152.23
- MDL Number:
- MFCD00064149
- MOL File:
- 464-49-3.mol
- MSDS File:
- SDS
| Melting point | 178-182 °C (lit.) |
|---|---|
| alpha | D25 +41 to +43° (c = 10 in U.S.P. alcohol) according to U.S.P. specif |
| Boiling point | 204 °C |
| Density | 0,99 g/cm3 |
| vapor density | 5.24 (vs air) |
| vapor pressure | 4 mm Hg ( 70 °C) |
| refractive index | 44.5 ° (C=20, EtOH) |
| FEMA | 2230 | D-CAMPHOR |
| Flash point | 148 °F |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in water, very soluble in alcohol and in light petroleum, freely soluble in fatty oils, very slightly soluble in glycerol. |
| form | Crystals |
| color | White |
| Odor | at 10.00 % in dipropylene glycol. camphor minty phenolic herbal woody |
| Odor Type | camphoreous |
| explosive limit | 3.5% |
| optical activity | [α]25/D +44°, c = 10 in ethanol |
| Water Solubility | Soluble in water (0.1 g/L at 20°C). |
| Merck | 14,1732 |
| JECFA Number | 1395 |
| BRN | 2042745 |
| Stability | Stable. Incompatible with strong reducing agents, strong oxidizing agents, chlorinated solvents. Protect from direct sunlight. |
| LogP | 2.3 at 20℃ |
| CAS DataBase Reference | 464-49-3(CAS DataBase Reference) |
| Substances Added to Food (formerly EAFUS) | D-CAMPHOR |
| FDA 21 CFR | 172.515 |
| FDA UNII | N20HL7Q941 |
| EPA Substance Registry System | D-Camphor (464-49-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H228-H315-H318-H332-H371 | |||||||||
| Precautionary statements | P210-P280-P302+P352-P304+P340+P312-P305+P351+P338-P308+P311 | |||||||||
| Hazard Codes | F,Xn,Xi | |||||||||
| Risk Statements | 11-22-36/37/38-20/21/22-36 | |||||||||
| Safety Statements | 16-26-36-37/39 | |||||||||
| RIDADR | UN 2717 4.1/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | EX1260000 | |||||||||
| Autoignition Temperature | 870 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29142910 | |||||||||
| NFPA 704 |
|
D-CAMPHOR price More Price(42)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W223018 | D-Camphor ≥97%, FG | 464-49-3 | 1kg | $75.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | W223018 | D-Camphor ≥97%, FG | 464-49-3 | 10Kg | $513 | 2024-03-01 | Buy |
| Sigma-Aldrich | 50843 | D-Camphor analytical standard | 464-49-3 | 100mg | $99.5 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1087508 | Camphor United States Pharmacopeia (USP) Reference Standard | 464-49-3 | 1g | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM40374 | (1R)-(+)-Camphor solution certified reference material, 2000?μg/mL in methanol, ampule of 1?mL | 464-49-3 | 1mL | $109 | 2022-05-15 | Buy |
D-CAMPHOR Chemical Properties,Uses,Production
Description
(1R)-(+)-Camphor is a terpene that has been found in C. sativa, C. indica, and C. sativa/C. indica hybrid strains as well as the essential oils from a variety of herbs including rosemary, lavender, and sage and has diverse biological activities. It inhibits norepinephrine secretion and cytosolic calcium and sodium increases induced by the nicotinic acetylcholine receptor (nAChR) agonist 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) in chromaffin cells (IC50s = 70, 88, and 19 μM, respectively). (1R)-(+)-Camphor (65-260 μM) induces proliferation of and increases expression of collagen IA, collagen IIIA, collagen IVA, and elastin in human primary dermal fibroblasts. In vivo, (1R)-(+)-camphor increases expression of collagen IA, collagen IIIA, collagen IVA, and elastin in skin in UV-exposed mice when administered at doses of 26 and 55 mM in drinking water post UV-exposure. It reduces cough frequency in citric acid-challenged guinea pigs. (1R)-(+)-Camphor is insecticidal, reducing digging activity and inducing mortality of fire ant workers. It has also been used as a building block in the synthesis of cannabinergic ligands.
Chemical Properties
white crystals
Chemical Properties
d-Camphor has a warm, minty, almost ethereal diffusive aroma. For other details of description, see Camphor Tree.
Occurrence
Frequently occurring in nature as the d- or l-form; the optically inactive form is seldom encountered. The d-form has been reported found in Cinnamomum camphora Ness. (Laurus camphora L.) from China, Japan and the East Indies; in Sassafras officinale, Lavandula spica and in other Labiatae. The l-form is reported found in the essential oils of Salvia grandiflora, Matricaria parthenium, Artemisia herba alba; the optically inactive form is found in Chrysanthemum japonicum sinense. Also reported found in orange peel oil, lemon peel oil, apricot, raspberry, anise, cinnamon root bark, ginger, pepper, coriander, calamus, sweet fennel and rosemary.
Uses
D-Camphor may be used as reference material for the quantification of the analyte in Saraca asoca and coriander using chromatography techniques.
Uses
(1R)-(+)-Camphor is used as a chiral intermediate and auxiliary. It is also used as a skin antipruritic and as an anti-infective agent.
Uses
analgesic, antiinfective, antipruritic
Uses
(R)-(+)-Camphor is a terpenoid with a wide variety of use. (R)-(+)-Camphor has insecticidal activity and is also used as an antimicrobial agent. (R)-(+)-Camphor is used as a culinary flavouring agent in parts of asia.
Definition
ChEBI: The (R)- enantiomer of camphor.
Aroma threshold values
Detection at 1 to 1.29 ppm
Taste threshold values
Taste characteristics at 20 ppm: medicinal, camphoraceous, mentholic and woody.
General Description
Colorless or white crystals. Sublimes. Flash point 149°F. Burns readily with a bright, smoky flame. Penetrating aromatic odor. Pungent, aromatic taste followed by a sensation of cold.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
D-CAMPHOR is incompatible with strong oxidizing agents, strong reducing agents and chlorinated solvents.
Fire Hazard
D-CAMPHOR is combustible.
Flammability and Explosibility
Flammable
Synthesis
Natural camphor is obtained by distillation from the plants of Cinnamomum or Laurus camphora from China and Japan, together with the corresponding essential oils; the raw camphor contains several impurities. It is separated from the water and the essential oil by pressure or by centrifugation and subsequently purified by sublimation or crystallization. Synthetic camphor is prepared from pinene isolated by fractional distillation of turpentine oil; pinene is reacted to bornyl chloride with gaseous HCl under pressure and then to camphene. The distilled and purified camphene is then oxidized to camphor with Na+ or K+ bichromate in the presence of H2SO4.
Purification Methods
Crystallise it from EtOH, 50% EtOH/water, MeOH, or pet ether or from glacial acetic acid by addition of water. It can be sublimed (50o/14mm) and also fractionally crystallised from its own melt. It is steam volatile. It should be stored in tight containers as it is appreciably volatile at room temperature. The solubility is 0.1% (H2O), 100% (EtOH), 173% (Et2O) and 300%
D-CAMPHOR Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 747 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 | sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
Related articles
- D-camphor:a specific enantiomer of camphor
- D-Camphor: Versatile compound used in pharmaceuticals, fragrances, and plastics; handle with care due to potential toxicity an....
- Dec 20,2023
- Camphor, D-Camphor and L-Camphor
- Camphor is highly lipid soluble and easily crosses the blood–brain barrier. The natural form of camphor is D-camphor, while th....
- Nov 1,2023
View Lastest Price from D-CAMPHOR manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | D-CAMPHOR; Camphor tree extract
464-49-3
|
US $0.00 / KG | 1KG | ≥98% HPLC | 1000KG | Changsha Staherb Natural Ingredients Co., Ltd. | |
 |
2024-04-19 | D-CAMPHOR
464-49-3
|
US $6.00 / KG | 1KG | More than 99% | 2000KG/MONTH | Hebei Saisier Technology Co., LTD | |
 |
2024-04-16 | camphor
464-49-3
|
US $0.00-0.00 / kg | 25kg | 96.00% | 1000kg | Hebei ZB Gamay Biological Technology Co.,Ltd |
-

- D-CAMPHOR; Camphor tree extract
464-49-3
- US $0.00 / KG
- ≥98% HPLC
- Changsha Staherb Natural Ingredients Co., Ltd.
-

- D-CAMPHOR
464-49-3
- US $6.00 / KG
- More than 99%
- Hebei Saisier Technology Co., LTD
-

- camphor
464-49-3
- US $0.00-0.00 / kg
- 96.00%
- Hebei ZB Gamay Biological Technology Co.,Ltd
464-49-3(D-CAMPHOR)Related Search:
1of4