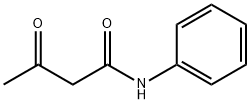
Acetoacetanilide
- CAS No.
- 102-01-2
- Chemical Name:
- Acetoacetanilide
- Synonyms
- AAA;3-OXO-N-PHENYLBUTANAMIDE;AAN;ethyl 3-oxo-3-(phenylamino)propanoate;Acetoacetanilid;n-phenylacetoacetamide;Linazol;usafek-1239;Coupler 633;USAF ek-1239
- CBNumber:
- CB0463919
- Molecular Formula:
- C10H11NO2
- Molecular Weight:
- 177.2
- MDL Number:
- MFCD00008780
- MOL File:
- 102-01-2.mol
- MSDS File:
- SDS
| Melting point | 83-88 °C (lit.) |
|---|---|
| Boiling point | 129°C 24mm |
| Density | 1.26 |
| vapor density | 6.1 (vs air) |
| vapor pressure | 0-0.013Pa at 20-50℃ |
| refractive index | 1.5168 (estimate) |
| Flash point | 325 °F |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 12.21±0.46(Predicted) |
| form | Crystalline Powder |
| color | White |
| Water Solubility | 5 g/L (20 ºC) |
| Merck | 14,58 |
| BRN | 473419 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| LogP | 0.93 at 23℃ |
| CAS DataBase Reference | 102-01-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | W35JB9PY3X |
| NIST Chemistry Reference | Butanamide, 3-oxo-N-phenyl-(102-01-2) |
| EPA Substance Registry System | Acetoacetanilide (102-01-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H341-H302-H312 | |||||||||
| Precautionary statements | P280f-P280-P201-P202-P308+P313-P405-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 21/22 | |||||||||
| Safety Statements | 36 | |||||||||
| RTECS | AK4200000 | |||||||||
| Autoignition Temperature | 843 °F | |||||||||
| HS Code | 2921419000 | |||||||||
| Toxicity | LD50 orl-rat: 5400 mg/kg LONZA# 08FEB79 | |||||||||
| NFPA 704 |
|
Acetoacetanilide Chemical Properties,Uses,Production
Chemical Properties
White to off-white powder
Uses
Acetoacetanilide is used as intermediate for the manufacture of organic pigments and dyestuffs.
Uses
Acetoacetanilide may be used to synthesize:
- azo pigments
- acetoacetanilido-4-aminoantipyrine (Schiff base)
- 6-aryl-2-methyl-4-oxo-N,N′-diphenyl-2-cyclohexene-1,3-dicarboxamides
- photoluminescent lanthanide complexes
Uses
Acetoacetanilide (AAA) flakes is used as an intermediate for the manufacture of organic pigments and dyestuffs. For further information consult the Product information sheet. Product Data Sheet
Synthesis Reference(s)
The Journal of Organic Chemistry, 56, p. 1713, 1991 DOI: 10.1021/jo00005a013
General Description
White crystalline solid.
Air & Water Reactions
Water insoluble.
Reactivity Profile
Organic amides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides with strong reducing agents. Amides are very weak bases (weaker than water). Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
ACUTE/CHRONIC HAZARDS: Acetoacetanilide is a weak allergen. When heated to decomposition it emits toxic fumes.
Fire Hazard
Acetoacetanilide is combustible.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. A weak allergen. See also ACETANILIDE. Combustible when exposed to heat or flame. See ANILINE and CYANIDE for disaster hazard. When heated to decomposition it emits toxic NOx, fumes. To fight fire, use alcohol foam, water mist, CO2, dry chemical.
Purification Methods
Crystallise the anilide from H2O, aqueous EtOH or pet ether (b 60-80o). [Williams & Krynitsky Org Synth Coll Vol III 10 1955.]
Acetoacetanilide Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
102-01-2(Acetoacetanilide)Related Search:
1of4