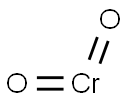chromium dioxide
- CAS No.
- 12018-01-8
- Chemical Name:
- chromium dioxide
- Synonyms
- CrO2;accroxr;accroxc;magtrieve;MAGTRIEVE(TM);chromiumdioxide;chromium(iv)dioxide;chromiumoxide(cro2);chromiumdioxide(cro2);Chromium oxide (CrO2)
- CBNumber:
- CB0721172
- Molecular Formula:
- CrO2
- Molecular Weight:
- 83.99
- MDL Number:
- MFCD00049888
- MOL File:
- 12018-01-8.mol
| Melting point | >375 °C (dec.)(lit.) |
|---|---|
| Boiling point | decomposes to Cr2O3℃ [KIR78] |
| Density | 4.85 g/mL at 25 °C(lit.) |
| solubility | insoluble in H2O; soluble in acid solutions |
| form | brown-black tetragonal powder |
| color | brown-black tetragonal powder |
| Water Solubility | insoluble H2O, soluble acids giving Cr+++and dichromate [KIR78] |
| Merck | 13,2254 |
| CAS DataBase Reference | 12018-01-8 |
| FDA UNII | 7BHJ7466GL |
| EPA Substance Registry System | Chromium oxide (CrO2) (12018-01-8) |
chromium dioxide price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Biosynth Carbosynth | FC168663 | Chromium dioxide | 12018-01-8 | 500g | $60 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FC168663 | Chromium dioxide | 12018-01-8 | 1kg | $100 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FC168663 | Chromium dioxide | 12018-01-8 | 2Kg | $150 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FC168663 | Chromium dioxide | 12018-01-8 | 5kg | $300 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FC168663 | Chromium dioxide | 12018-01-8 | 10Kg | $450 | 2021-12-16 | Buy |
chromium dioxide Chemical Properties,Uses,Production
Chemical Properties
dark brown or black powder(s); tetr; used in magnetic tapes [KIR78]; ferromagnetic; rutile structure [MER06]
Physical properties
Chromium dioxide [12018-01-8], chromium(IV)oxide, CrO2, is a ferromagnetic material with a specific saturation magnetization Ms/? of 132 A m2 kg?1 at 0 K, corresponding to the spin of two unpaired electrons per Cr4+ ion. TheMs/n value of CrO2 at room temperature is ca. 100 A m2 kg?1; CrO2 magnetic pigments reach values of 77–92 A m2 kg?1. The material crystallizes with a tetragonal rutile lattice in the form of small needles, which have the desired magnetic shape anisotropy. The morphology of the particles can be varied with several dopants, particularly antimony and tellurium. The coercive field strength can be controlled between ca. 30 and 75 kA m?1 (in addition to shape) by doping with transition metal ions, which modify the magneto-crystalline anisotropy of the material; the Fe3+ ion being industrially important.
Uses
Facilitates high yield aromatization of Hantzsch 1,4-dihydropyridines to the corresponding pyridines.
Uses
In magnetic recording tapes; as catalyst.
Uses
Chromium dioxide is used exclusively for magnetic recording media. It may also be used in combination with cobalt-modified iron oxides in the production of magnetic recording media. The world consumption of CrO2 is decreasing continuously as compact disc and digital videodisc are taking over the audio and video market. The sole producer is DuPont.
In the course of the development of pigments for magnetic information storage, CrO2 was the first widely used pigment material that gave a higher recording density than ã-Fe2O3. In the field of audio recording this led to the IEC II standard or “chrome position”.
Production Methods
The conversion of an intimate mixture of Cr(III) and Cr(VI) compounds into CrO2 under hydrothermal conditions has been developed into an industrial process in autoclaves at ca. 350 °C and 300 bar.
Pure CrO2 slowly disproportionates in the presence of water. The CrO2 crystal surface of commercial pigments is therefore topotactically converted to â-CrOOH, which serves as a protection layer. In the absence of moisture, CrO2 is stable up to ca. 400 °C, above this temperature it decomposes to form Cr2O3 and oxygen.
chromium dioxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ShanDong Look Chemical Co.,Ltd. | +8617653113219 | sales01@sdlookchemical.com | China | 2739 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 50000 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |