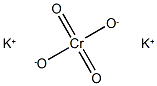
Potassium chromate
- CAS No.
- 7789-00-6
- Chemical Name:
- Potassium chromate
- Synonyms
- CHROMATE;Chromium(VI);KrCrO4;BETZ 0213;tarapacaite;Chromate, CrO;chromateofpotash;chromateofpotass;CHROMATE STANDARD;potassiumchromate6
- CBNumber:
- CB6398284
- Molecular Formula:
- CrK2O4
- Molecular Weight:
- 194.1903
- MDL Number:
- MFCD00011368
- MOL File:
- 7789-00-6.mol
- MSDS File:
- SDS
| Melting point | 971 °C (lit.) |
|---|---|
| Density | 1.00 g/mL at 20 °C |
| vapor density | 6.7 (vs air) |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | Solid |
| Specific Gravity | 2.732 |
| color | Yellow |
| PH | 9.0-9.8 (50g/l, H2O, 20℃) |
| Water Solubility | 640 g/L (20 ºC) |
| Merck | 14,7622 |
| Exposure limits |
ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
| Stability | Stable. Strong oxidizing agent - contact with combustible materials may lead to fire or violent reaction. Incompatible with strong reducing agents, combustible materials. |
| InChIKey | XMXNVYPJWBTAHN-UHFFFAOYSA-N |
| CAS DataBase Reference | 7789-00-6(CAS DataBase Reference) |
| EWG's Food Scores | 5 |
| FDA UNII | 5P0R38CN2X |
| EPA Substance Registry System | Potassium chromate(VI) (7789-00-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H317-H319-H335-H340-H350i-H410 | |||||||||
| Precautionary statements | P202-P273-P280-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | T,N,O | |||||||||
| Risk Statements | 49-46-43-51/53-8-50/53-36/37/38-22-45-52/53-25-42/43-20 | |||||||||
| Safety Statements | 53-45-60-61-36/37-26-23 | |||||||||
| RIDADR | UN 3288 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GB2940000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28415000 | |||||||||
| NFPA 704 |
|
Potassium chromate price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 12249 | Potassium chromate puriss., ≥99% | 7789-00-6 | 100g | $61.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 12249 | Potassium chromate puriss., ≥99% | 7789-00-6 | 1kg | $339 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04952 | Potassium chromate for analysis EMSURE? ACS,Reag. Ph Eur | 7789-00-6 | 250g | $193 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.04952 | Potassium chromate for analysis EMSURE? ACS,Reag. Ph Eur | 7789-00-6 | 1kg | $476 | 2024-03-01 | Buy |
| Alfa Aesar | 042564 | Chromate | 100mL | $124 | 2024-03-01 | Buy |
Potassium chromate Chemical Properties,Uses,Production
Inorganic compound
Potassium chromate, commonly known as tarapacaite, is an inorganic compound, which is yellow orthorhombic or hexagonal crystal at room temperature. The relative density is 2.732, and the melting point is 968 °C. It is toxic and can disolve in water to form alkaline chromate ion hydrolysis solution. And it is insoluble in alcohol and ether. After added acid, the yellow solution of potassium chromate will turn orange, which is the color of dichromate. There is an equilibrium between the conversion of chromate and dichromate in the solution: 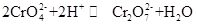
When the acid is added to the potassium chromate solution, the equilibrium will shift toward the direction of generating potassium dichromate, and when the alkali is added to the potassium dichromate solution, the equilibrium will shift to the direction of generating potassium chromate. Potassium chromate has oxidative properties, and it can react with the reducing agent in alkaline medium to form Cr(OH)4-(namely CrO¬2-). Adding different solutions respectively containing barium ion, lead ion and silver ion to the potassium chromate solution will form the corresponding insoluble chromate: barium chromate BaCrO4 (yellow), lead chromate pbCrO4 (yellow), silver chromate Ag2CrO4(Brick red). And the presence of chromate ions can be demonstrated by the characteristic color of these insoluble chromates. Potassium chromate can be used as analytical reagent, oxidant, mordant, metal rust inhibitor, and used for barium and silver trace analysis. It can also be used as raw materials of tanning and medicine industry and other chromium compounds. Chromite Fe(CrO2)2 powder can be used as raw material and calcined with potassium hydroxide, limestone (dolomite) in the air, and then extracted with potassium sulfate solution to obtain potassium chromate.
The above information is edited by Xiao Nan of Chemicalbook.
Potassium chromate indicator method
Potassium chromate indicator method, also known as Moore (Mohr), is a precipitation titration method (silver method) that uses potassium chromate (K2CrO4) as indicator and silver nitrate (AgNO3) as the standard solution. This method is mainly used for the determination of chlorine Ion (Cl-) or bromide ion (Br-). Add a small amount of K2CrO4 as indicator before stating determination, and then titrate with AgNO3 standard solution. After the start of the titration, the precipitate of white (silver chloride) or pale yellow (silver bromide) precipitates first. When Cl-or Br-precipitates quantitatively, a little excess silver nitrate solution will cause the concentration of Ag+ suddenly increasing to immediately generate brick red silver chromate (Ag2CrO4) precipitation, indicating the titration endpoint. The amount of indicator and the acidity of the solution are two major problems of this titration method. If the K2CrO4 concentration is too high, the color of the titration solution will be too deep to hinder the observation of Ag2CrO4 precipitation color in the end; if the K2CrO4 concentration is too low, an excessively considerable amount of silver nitrate solution will be needed after the quantitative precipitation of silver halide to generate silver chromate precipitation to instruct the titration end point, which will results the titration error increasing. When the 0.1mol/l AgNO3 solution is used to titrate the 0.1mol/l halide, if the concentration of K2CrO4 is 5× 10-3mol/l, the end point error is only +0.06%, which can be thought that the accuracy of the analysis result is not affected. K2CrO4 indicator method can not be carried out in acidic or alkaline solution, because K2CrO4 will be converted to potassium dichromate (K2Cr2O7) at a small pH value, and Ag+ will precipitate in the form of silver oxide (Ag2O) when the pH is too high. Commonly, the suitable acidity range is pH = 6.5~ 10.5, but when there are ammonium salts in solution, the solution acidity pH = 6.5~7.2 is appropriate.
The potassium chromate indicator method can only be used for the direct titration of Cl-or Br-ions, and the titration result is their total when coexisting. This method is not suitable for the determination of iodide ion (I-) or thiocyanate ion (SCN-), because they are too easily absorbed by sedimentation and the end point is unclear. This method is also not suitable for titrating Ag+ with Cl-, but Ag+ can be determined using the back titration, namely add an excessive amount of NaCl standard solution in the test solution, and then use AgNO3 standard solution to titrate excess Cl-ions. In the solution, all the cations that can form precipitates with CrO2-4 or the anions that can precipitate with Ag+ will interfere with the determination. Potassium chromate indicator method is mainly used for the determination of the Cl-ions in very dilute solution, such as the determination of Cl-in drinking water and industrial products impurities.
Potassium dichromate
Potassium dichromate, also known as red alum potassium, is orange-red triclinic crystal or needle-like crystal. The density is 2.676 g/cm3. The melting temperature is 398 °C. It is soluble in water and insoluble in ethanol. It has strong oxidizing property and decomposes at 1300 °C.
In production, potassium dichromate is always used to introduce chromium to make the enamel colored when melting. It is the coloring agent of colored titanium milk yellow glaze and titanium yellow glaze, and the used dosage is generally 0.06%~0.12%. Potassium dichromate and copper oxide can also be mix-used to obtain green, bamboo green, fruit green and other color glaze. However, these colored glazes are generally less glossy due to the influence of chromate.
The color glaze obtained from chromium oxide or potassium (sodium) dichromate often is yellow-green due to the valence change of chromium ions in the melting process. Potassium dichromate is also commonly used to make various green pigments and pink pigments.
Potassium dichromate should meet the required targets: potassium dichromate content ≥ 99%, chloride (Cl) content ≤ 0.08%, water insoluble ≤ 0.05%.
The above information is compiled by Yaoyao of Chemicalbook.
Solubility in water (g/100ml)
Dissolved grams per 100 ml of water at different temperatures (°C):
60 g/10 °C; 63.7 g/20 °C; 66.7 g/30 °C; 67.8 g/40 °C
70.1 g/60 °C; 74.5 g/90 °C
Toxicity
See sodium chromate
Chemical properties
Lemon yellow orthorhombic crystal; Soluble in water; insoluble in alcohol
Application
Used as analytical reagent, oxidant, mordant and metal rust inhibitor;used for the manufacture of chromate,used as oxidant and mordant of printing and dyeing. Used for ink, paint, enamel, metal corrosion and so on,mainly used in the manufacture of chemical reagents and pigments.
Preparation
Neutralization method: Dissolve potassium dichromate in the mother liquor and water, and then add the mixture to the reactor. Next add potassium hydroxide under stirring to carry out neutralization reaction. The produced potassium chromate is weakly alkaline, and then evaporated for concentration, cooled for crystallization, separated and dried to obtain the finished potassium chromate products.
K2Cr2O7 + 2KOH → 2K2CrO4 + H2O
The separated mother liquor will be returned to the dissolving step for dissolving potassium dichromate.
Chemical Properties
Potassium chromate(VI) is a yellow crystalline solid.
Chemical Properties
lemon-yellow crystals
Uses
It is used as an oxidizing agent.
Uses
Potassium chromate (K2CrO4) is soluble in water and is used to make bright yellow inks and paint pigments. It is also used as a reagent in chemical laboratories and as a mordant to “fix” dyes in colored textiles.
Uses
Has a limited application in enamels, finishing leather, rustproofing of metals, being replaced by the sodium salt; as reagent in analytical chemistry.
Definition
A salt containing the ionCrO42-.
Definition
potassium chromate: A bright yellowcrystalline solid, K2CrO4, solublein water and insoluble in alcohol;rhombic; r.d. 2.73; m.p. 968.3°C; decomposes without boiling. It is producedindustrially by roasting powderedchromite ore with potassiumhydroxide and limestone and leachingthe resulting cinder with hotpotassium sulphate solution. Potassiumchromate is used in leatherfinishing, as a textile mordant, and inenamels and pigments. In the laboratoryit is used as an analyticalreagent and as an indicator. Likeother chromium(III) compounds it istoxic when ingested or inhaled.
Definition
ChEBI: A potassium salt consisting of potassium and chromate ions in a 2:1 ratio.
General Description
Potassium chromate is a yellow crystalline solid. Potassium chromate is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Potassium chromate is used in chemical analysis, in making pigments for paints and inks, as a fungicide, and to make other chromium compounds.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Oxidizing agents, such as Potassium chromate , can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Inhalation causes local irritation of mucous membranes; continuing nose irritation can result in perforation of nasal septum. Ingestion may cause violent gastroenteritis, circulatory collapse, vertigo, coma, and toxic nephritis; ingestion of excessive quantities can be fatal. Contact with eyes causes severe irritation and conjunctivitis. Repeated or prolonged exposure to dust, mist, or solutions may cause dermatitis; contact with breaks in the skin may cause ``chrome sores'' appearing as slow-healing, hard-rimmed ulcers which leave the area vulnerable to infection.
Fire Hazard
Behavior in Fire: May increase intensity of fire if in contact with combustible materials. Cool containers and spilled material with plenty of water.
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. Poison by ingestion, intravenous, subcutaneous, and intramuscular routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A powerful oxidizer. When heated to decomposition it emits toxic fumes of K2O. Used as a mordant for wool, in the oxidizing and treatment of dyes on materials. See also CHROMIUM COMPOUNDS.
Potential Exposure
Potassium chromate is used in printing: photomechanical processing; chrome-pigment production; and wool preservative methods; to make dyes, pigments, inks and enamels; as an oxidizing agent; analytical reagent; in electroplating; explosives.
Shipping
UN1479 Oxidizing solid, n.o.s., Hazard Class: 5.1; Labels: 5.1-Oxidizer, Technical Name Required. UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Crystallise it from conductivity water (0.6g/mL at 20o), and dry it between 135o and 170o.
Incompatibilities
A powerful oxidizer. Violent reactions with combustibles, organics, powdered metals; or easily oxidizable substances. Contact with hydroxylamine, hydrazine causes explosion.
Potassium chromate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12465 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21666 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 255 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | sales@tnjchem.com | China | 34571 | 58 |
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2136 | 58 |
View Lastest Price from Potassium chromate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-11 | Potassium chromate
7789-00-6
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2021-10-19 | Potassium chromate
7789-00-6
|
US $10.00 / KG | 1KG | 99% | 20 tons | Hebei Zhanyao Biotechnology Co. Ltd | |
 |
2021-09-15 | Potassium chromate
7789-00-6
|
US $10.00 / KG | 100KG | 99% | 100 mt | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Potassium chromate
7789-00-6
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Potassium chromate
7789-00-6
- US $10.00 / KG
- 99%
- Hebei Zhanyao Biotechnology Co. Ltd
-

- Potassium chromate
7789-00-6
- US $10.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
7789-00-6(Potassium chromate )Related Search:
1of4