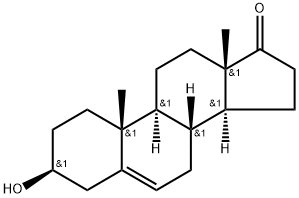
Dehydroepiandrosterone
- CAS No.
- 53-43-0
- Chemical Name:
- Dehydroepiandrosterone
- Synonyms
- DHEA;PRASTERONE;DEHYDROISOANDROSTERONE;DEHYDROEPIANDOSTERONE;TRANS-DEHYDROANDROSTERONE;Dehydroepiandrosterone(DHEA);ANDROSTENOLONE;dehydroepiandrosteron;3β-Hydroxyandrost-5-en-17-one;3BETA-HYDROXYANDROST-5-EN-17-ONE
- CBNumber:
- CB1475670
- Molecular Formula:
- C19H28O2
- Molecular Weight:
- 288.43
- MDL Number:
- MFCD00003613
- MOL File:
- 53-43-0.mol
- MSDS File:
- SDS
| Melting point | 149-151 °C(lit.) |
|---|---|
| Boiling point | 370.65°C (rough estimate) |
| alpha | 12 º (c=2, ethanol 96% 25 ºC) |
| Density | 1.0484 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| Flash point | 9℃ |
| storage temp. | Hormones |
| solubility | insoluble in H2O; ≥13.7 mg/mL in DMSO; ≥58.6 mg/mL in ETOH |
| form | Fine Crystalline Powder |
| pka | 15.02±0.60(Predicted) |
| color | White |
| Water Solubility | 21.8mg/L(23.5 ºC) |
| Merck | 2871 |
| BRN | 2058110 |
| InChIKey | FMGSKLZLMKYGDP-USOAJAOKSA-N |
| LogP | 3.230 |
| CAS DataBase Reference | 53-43-0 |
| EWG's Food Scores | 1-3 |
| NCI Dictionary of Cancer Terms | dehydroepiandrosterone; DHEA |
| FDA UNII | 459AG36T1B |
| NIST Chemistry Reference | 5-Androstene-3«beta»-ol-17-one(53-43-0) |
| NCI Drug Dictionary | prasterone |
| ATC code | A14AA07,G03XX01 |
| EPA Substance Registry System | Prasterone (53-43-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361fd |
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 |
| Hazard Codes | Xi,T,F |
| Risk Statements | 36/37/38-39/23/24/25-23/24/25-11 |
| Safety Statements | 26-36-24/25-45-36/37-16-7 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 2 |
| RTECS | BV8396000 |
| HS Code | 29372900 |
Dehydroepiandrosterone price More Price(53)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | D-063 | Dehydroepiandrosterone (DHEA) solution 1.0?mg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 53-43-0 | 1mL | $116 | 2024-03-01 | Buy |
| Sigma-Aldrich | 252805 | DHEA - CAS 53-43-0 - Calbiochem Major secretory steroidal product of the adrenal gland. | 53-43-0 | 10g | $251 | 2024-03-01 | Buy |
| Cayman Chemical | 15728 | Dehydroepiandrosterone ≥97% | 53-43-0 | 1g | $44 | 2024-03-01 | Buy |
| Sigma-Aldrich | D4000 | trans-Dehydroandrosterone ≥99% | 53-43-0 | 10g | $166 | 2024-03-01 | Buy |
| Sigma-Aldrich | D4000 | trans-Dehydroandrosterone ≥99% | 53-43-0 | 25g | $371 | 2024-03-01 | Buy |
Dehydroepiandrosterone Chemical Properties,Uses,Production
description
Dehydroepiandrosterone (DHEA) is a steroid hormone that is a popular nonprescription oral “dietary supplement” used by men to enhance cognitive function, mood, libido, and athletic performance. Before 1994, DHEA was a prescription medicine. After the passage of the Dietary Supplement Health and Education Act of 1994, DHEA was reclassified as a nutritional supplement. Although sold over the counter in 25- and 50-mg strengths, DHEA is widely touted to maximize results at doses of 200 mg or higher. DHEA is banned by the International Olympic Committee and the National Collegiate Athletic Association as an anabolic agent. Limited information is available regarding potential harmful effects resulting from its supplemental use.
Indications and Usage
Dehydroepiandrosterone (DHEA,) chemical name 3β-hydroxy-5alpha-androstane-17-ketone, is an esterifying 3–β–hydroxy steroid retaining 5,6 cholesterol. A white crystalline powder, soluable in ethanol, ether, and benzene, and slightly soluable in chroloform and petroleum ether. Precipitates in digitalis.
DHEA is an estrogen precursor secreted by the reticular layer of the human adrenal cortex. Prevents obesity, resists diabetes, fights cancer, fights cortical disease, and delays senility treats immune deficiencies, promotes the growth and differentiation of bone cells, and promotes the synthesis of protein. It also resists viral infections, improves memory, and relieves nervous tension. DHEA is the main ingredient in steroid hormone intermediates (such as norethindrone and bisacetylene, etc.) and in birth control, and is involved in the secretion of many adrenal hormones. It has undergone extensive clinical research on treating menopausal syndrom, chorionitis, coronary heart disease, gout, psoriasis, AIDS and so on.
Mechanisms of Action
DHEA has a thyroid stimulating effect inhibiting food and fat intake and reducing fat accumulation, etc. It improves glucose tolerance, increases insulin level and fights diabetes. It can enhance endocrine system actiity, reduce cortisol levels, and resist a variety of pathological processes. It can help the body obtain cortical antibodies. DHEA has a strong protective and synergistic effect when used to treat tumors, becuase it inhibits ribose 5-phosphate. Inhibits cancer by inhibiting excessive mitochondria (NADPH) and ribose 5-phosphate esters. Regulates the growth of pancreatic cancer cells by regulating the concentration of estrogen in blood plasma. A decline in GnRH gene expression leads to aging, and DHEA can restore GnRH neuronal activity, stopping or improving certain diseases associated with declines in DHEA by stimulating GnRH biosynthesis. Restores impaired immune response, improves T- and B-cell function, and plays an important role in enhancing the physiological activity of insulin-like growth factor (IGF-1,) and is a potentially useful drug for immunodeficiency. DHEA alone cannot directly affect the growth and differential of osteoblasts, but it can do so by influencing 1,25-dihydroxyvitamin D3. The effects of DHEA on bone mass depend on the presence and form of sex hormones in bone cells and their endrocine effects on osteoblasts. DHEA is an anabolic protein hormone which promotes protein synthesis. According to the findings of Marrero and others, feeding DHEA (0.45%) to mice increased liver weight, increasing liver mitochondria by guiding liver protein restoring RNA and protein synthesis.
Description
Dehydroepiandrosterone(DHEA) is an endogenous steroid hormone that is secreted primarily by the adrenal gland and is the most abundant sex steroid. It is a neurohormone; small quantities of DHEA are produced in the brain and declines in serum and CSF with age. (Knopman and Henderson, 2003). Metabolites of DHEA include androstenedione , which subsequently may be metabolized to testosterone or estrone which is an estradiol precursor. In addition to serving as an intermediate in the biosynthesis of sex steroids, DHEA directly modulates a number of cellular and nuclear receptors.
Chemical Properties
white fine crystalline powder. There are two crystal forms. Needle-like crystal, melting point 140-141oC; small leaf-like crystal, melting point 152-153oC. Has right-handedness. Soluble in alcohol, ether, benzene, slightly soluble in chloroform, petroleum ether. In case of digitonin to produce precipitation.
Originator
Aslera ,Genelabs Technologies, Inc.
Occurrence
DHEA is naturally occurring in yam (see Wild Yam, p. 596-597).
Uses
Dehydroepiandrosterone (DHEA) is a major C19 steroid produced by the adrenal cortex. It is a major secretory steroidal product of the adrenal gland; secretion progresively declines with aging. May have estrogen-or androgen-like effects depending on the hormonal milieu. Intracellularly converted to androstenedione. It is used in treatment of menopausal syndrome. People living with HIV may be interested in taking DHEA for a variety of reasons, including treatment of depression, increasing bone density, decreasing arterial plaques, improvement of immune function with HIV, increased memory, and increased muscle strength.
Definition
ChEBI: Dehydroepiandrosterone is an androstanoid that is androst-5-ene substituted by a beta-hydroxy group at position 3 and an oxo group at position 17. It is a naturally occurring steroid hormone produced by the adrenal glands. It has a role as an androgen, a human metabolite and a mouse metabolite. It is a 17-oxo steroid, an androstanoid and a 3beta-hydroxy-Delta(5)-steroid.
Manufacturing Process
To a solution of 1 gram of 16-dehydropregnenolon-3β-acetate in 10 ml pyridine is added 0.22 gram of hydroxylamine hydrochloride, and the mixture is allowed to stand at room temperature for four days. One gram of 16dehydropregnenolon-3β-acetate oxime is dissolved in 30 ml of hot dioxane, and then the solution is cooled in an ice bath until about one-half of the dioxane has solidified. Then 1 gram of phosphorus pentachloride is added and the mixture is shaken until all the dioxane has melted. The mixture is maintained at 35°C, for seventy-five minutes, then an excess of ice is added and the solution is again allowed to stand at 35°C. After about thirty minutes, a solution of 5 ml of concentrated hydrochloric acid in 10 ml of water is added, and the mixture is diluted with water, extracted with ether and the ethereal extract washed with dilute sodium hydroxide solution. The ether is removed on a steam bath and the residue is worked up to yield dehydroepiandrosterone.
brand name
17-chetovis 17-hormoforin;Cetavister;Climatost;Dastonil;Dha-s (prasterone);Gynodian;Longevital 5000;Maxepa;Mentalormon;Mylis;Neurocotex;Psicosterone;Ro 66827;Sh 833;Ultrapla.
Therapeutic Function
Glucocorticoid
World Health Organization (WHO)
The World Health Organization has no information further to the above regarding preparations containing prasterone or to indicate that such preparations remain available.
General Description
DHEA is a major secretory steroidal product of the adrenal gland and is a hormonal precursor to both androgens and estrogens. It can also be synthesized using wild yam or soy, but there is no evidence to show that humans are able to increase DHEA levels by consuming these foods. DHEA and its sulfated metabolite, DHEA-S, are negative modulators of GABAA receptors.
Health Hazard
An experimental teratogen.Experimental reproductive effects. Questionablecarcinogen with experimental neoplastigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes.
Biochem/physiol Actions
Product does not compete with ATP.
Side effects
Side effects may include acne, skin rash, GI upset, hirsuitism, hypertension, and increased HDL. In people with HIV, additional side effects may include fatigue, nasal congestion, and headaches.
Safety
Dehydroepiandrosterone should always be used under the supervision of a medical professional. It is likely safe for people with low DHEA levels to take oral supplements short-term (<6 months) to restore DHEA to normal, but long-term use and doses resulting in high DHEA levels are possibly unsafe. Side effects are often seen with higher doses and longterm use.
target
Estrogen receptor | Progestogen receptor
References
[1]. kimonides vg, khatibi nh, svendsen cn, et al. dehydroepiandrosterone (dhea) and dhea-sulfate (dheas) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. proc natl acad sci u s a, 1998, 95(4): 1852-1857.
[2]. suzuki m, wright ls, marwah p, et al. mitotic and neurogenic effects of dehydroepiandrosterone (dhea) on human neural stem cell cultures derived from the fetal cortex. proc natl acad sci u s a, 2004, 101(9): 3202-3207.
[3]. charalampopoulos i, tsatsanis c, dermitzaki e, et al. dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic bcl-2 proteins. proc natl acad sci u s a, 2004, 101(21): 8209-8214.
Dehydroepiandrosterone Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of2
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 | sales06@tgybio.com | China | 959 | 58 |
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9339 | 58 |
| Joyochem Co.,Ltd | +86-0531-82687558 +8613290333633 | sales@joyochem.com | China | 42 | 58 |
| Senova Technology Co. Ltd. | +86-0755-86703119 +8618503098836 | info@senovatech.com | China | 349 | 58 |
| Pharmaceuticals Group Corp., Ltd. | +8613004379774 | wu@jiaerke.com | China | 33 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | 13119157289@163.com | China | 2971 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 | admin@firsky-cn.com | China | 436 | 58 |
Related articles
- Biological functions and use of Dehydroepiandrosterone (DHEA)
- Dehydroepiandrosterone (DHEA) and its sulfate ester, DHEAS, are the most abundant steroids circulating in human blood.
- Jun 16,2022
- How is dehydroepiandrosterone controlled?
- Dehydroepiandrosterone is an important precursor hormone, and is the most abundant circulating steroid present in the human bo....
- Oct 23,2019
- Application and Special precautions of DHEA
- DHEA is a hormone that is naturally made by the body. DHEA works in the body to make other male and female sex hormones within....
- Oct 18,2019
View Lastest Price from Dehydroepiandrosterone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-27 | Dehydroepiandrosterone
53-43-0
|
US $9.90-300.00 / g | 10g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-27 | Dehydroepiandrosterone
53-43-0
|
US $9.90-300.00 / g | 10g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-26 | Dehydroepiandrosterone
53-43-0
|
US $90.00-80.00 / kg | 0.10000000149011612kg | 99% | 100 tons | Hebei Kangcang new material Technology Co., LTD |
-

- Dehydroepiandrosterone
53-43-0
- US $9.90-300.00 / g
- 99%
- Wuhan Cell Pharmaceutical Co., Ltd
-

- Dehydroepiandrosterone
53-43-0
- US $9.90-300.00 / g
- 99%
- Wuhan Cell Pharmaceutical Co., Ltd
-

- Dehydroepiandrosterone
53-43-0
- US $90.00-80.00 / kg
- 99%
- Hebei Kangcang new material Technology Co., LTD