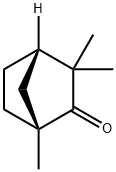
(-)-FENCHONE
- CAS No.
- 7787-20-4
- Chemical Name:
- (-)-FENCHONE
- Synonyms
- L-FENCHONE;(-)-FENCHONE;L(-)-FENCHONE;FENCHONE, (-)-;ALPHA-FENCHONE;laevo-fenchone;(-)-Fenchone>L-ALPHA-FENCHONE;(1R,4S)-fenchone;L-FENCHONE 98+%
- CBNumber:
- CB2283113
- Molecular Formula:
- C10H16O
- Molecular Weight:
- 152.23
- MDL Number:
- MFCD00151104
- MOL File:
- 7787-20-4.mol
- MSDS File:
- SDS
| Melting point | 5-6 °C(lit.) |
|---|---|
| Boiling point | 192-194 °C(lit.) |
| alpha | [α]D20 -50~-60° (c=4, C2H5OH) |
| Density | 0.948 g/mL at 25 °C(lit.) |
| vapor pressure | 2.149hPa at 20℃ |
| FEMA | 4519 | L-FENCHONE |
| refractive index |
n |
| Flash point | 127 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethanol (Slightly, Sonicated) |
| form | Liquid |
| color | Clear colorless to light yellow |
| Odor | at 100.00 %. camphor herbal earthy woody |
| Odor Type | camphoreous |
| optical activity | [α]24/D 50.5°, neat |
| Water Solubility | 1.983g/L at 20℃ |
| JECFA Number | 2200 |
| BRN | 2042710 |
| Dielectric constant | 12.0(20℃) |
| LogP | 2.54 at 20℃ |
| CAS DataBase Reference | 7787-20-4(CAS DataBase Reference) |
| Substances Added to Food (formerly EAFUS) | L-FENCHONE |
| EWG's Food Scores | 1 |
| FDA UNII | K6G5Y2Y3Q2 |
| EPA Substance Registry System | Bicyclo[2.2.1]heptan-2-one, 1,3,3-trimethyl-, (1R,4S)- (7787-20-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H411 | |||||||||
| Precautionary statements | P273-P391-P501 | |||||||||
| Risk Statements | 10 | |||||||||
| Safety Statements | 23-24/25 | |||||||||
| RIDADR | UN 1224 3/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RB7875000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29142900 | |||||||||
| Toxicity | The acute oral LD50 value in rats was reported as 616 g/kg (Jenner, Hagan, Taylor. Cook & Fitzhugh, 1964) and the acute dermal LD50 value in rabbits exceeded 5 g/kg (Leven-stein, 1975). | |||||||||
| NFPA 704 |
|
(-)-FENCHONE price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | W507709 | L-Fenchone ≥98% | 7787-20-4 | 100g | $74.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | W507709 | L-Fenchone ≥98% | 7787-20-4 | 5kg | $716 | 2024-03-01 | Buy |
| Sigma-Aldrich | CRM40762 | L-(-)-Fenchone solution certified reference material, 2000?μg/mL in methanol, ampule of 1?mL | 7787-20-4 | 1mL | $67.2 | 2024-03-01 | Buy |
| Sigma-Aldrich | 92101 | (?)-Fenchone analytical standard | 7787-20-4 | 50mg | $77 | 2024-03-01 | Buy |
| Sigma-Aldrich | 196436 | (1R)-(?)-Fenchone ≥98% | 7787-20-4 | 50g | $56.7 | 2024-03-01 | Buy |
(-)-FENCHONE Chemical Properties,Uses,Production
Chemical Properties
CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID
Occurrence
Reported to be found in many essential oils, including those of Thuja plicata, T.occiden-talis, T.standi shii, Russian anise, fennel, a few Artemisia varieties (A. frigida, A . verlotorum and A . santolinaefolia), Lavandula stoechas and L. burmannii. The highest levels (12-19%) are found in fennel oil (Fenarolis Handbook of Flavor Ingredients, 1975; Gildemeister & Hoffman, 1963).
Uses
Flavoring.
Uses
(1R,?4S)?-1,?3,?3-?Trimethylbicyclo[2.2.1]?heptan-?2-?one also known more commonly as (-)-Fenchone is a chiral intermediate of Fenchone and is currently being used for studies ranging from inhibitory effects of monoterpenes on human TRPA1 and odorant receptor of the malaria vector Anopheles gambiae.
Preparation
By isolation from cedar leaf oil (Thuja oil) or by various synthetic methods (Arctander, 1969).
Definition
ChEBI: A fenchone that has (1R,4S)-stereochemistry. It is a constituent of the essential oils obtained from fennel.
General Description
(1R)-(-)-Fenchone is a bridged bicyclic ketone found in fennel oil and thuja oil.
Flammability and Explosibility
Not classified
Pharmacology
In mice, fenchone injected sc in sesame oil produced clonic convulsions at non-lethal doses, with a median convulsive dose (CD50) of 1133 mg/kg, and a dose of 500 mg/kg given sc was an effective arousal agent, reducing the hexobarbitone sleep time (Wenzel & Ross, 1957). In rats, an ip dose of 500 mg/kg had no effect on pentobarbitone-depressed respiration, while ip doses of 50-400 mg/kg increased running activity but did not affect total activity (Wenzel & Ross, 1957). Fenchone showed some antispasmodic action on excised mouse intestine (Haginiwa, Harada & Morishita, 1963), and at 260 mmol/kg showed good choleretic properties of moderately long duration when given orally in olive oil to rats (M?rsdorf, 1966). Thomas (1958) reported that it acted as a central nervous system stimulant. Fenchone has been used medically as a counter-irritant (Merck Index, 1968).
Metabolism
Rimini (1901 & 1909) showed that, in the dog, fenchone was probably oxidized to 4-hydroxyfenchone. Reinartz & Zanke (1936) showed that there were other products. They separated, as lead salts, the glucuronides from the urine of dogs receiving d-fenchone. The lead was removed with sulphuric acid and the resulting solution was hydrolysed. In the resulting mixture of hydroxyfen chones, the presence of 4- and 5-hydroxyfenchones and 7r-apofenchone-3-carboxylic acid was demon strated (Williams, 1959).
Purification Methods
Purification is as for the (+)-enantiomer above and should have the same physical properties except for opposite optical rotations. UV has max 285nm ( 12.29). [Braun & Jacob Chem Ber 66 1461 1933, UV: Ohloff et al. Chem Ber 90 106 1957.] [Beilstein 7 III 392, 7 IV 212.]
(-)-FENCHONE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| Wuhan ChemNorm Biotech Co.,Ltd. | +86-27-8439 4403 18971486879 | sales@chemnorm.com | CHINA | 2935 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
View Lastest Price from (-)-FENCHONE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-11 | (-)-FENCHONE
7787-20-4
|
US $0.00 / kg | 1kg | 99% | 1000tons | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-04-07 | (-)-FENCHONE
7787-20-4
|
US $0.00 / KG | 1KG | 99% | 100 MT | Hebei Guanlang Biotechnology Co., Ltd. | |
 |
2023-02-24 | (-)-Fenchone
7787-20-4
|
US $0.00 / ml | 0.1ml | GC≥98% | 100 ml | Shanghai Standard Technology Co., Ltd. |
-

- (-)-FENCHONE
7787-20-4
- US $0.00 / kg
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- (-)-FENCHONE
7787-20-4
- US $0.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
-

- (-)-Fenchone
7787-20-4
- US $0.00 / ml
- GC≥98%
- Shanghai Standard Technology Co., Ltd.
7787-20-4((-)-FENCHONE)Related Search:
1of4