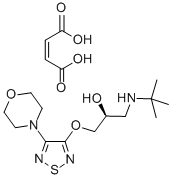
(S)-Timolol maleate
- CAS No.
- 26921-17-5
- Chemical Name:
- (S)-Timolol maleate
- Synonyms
- TIMOLOL MALEATE;Timolol maleate CRS;TimoL;timoptic;2-Propanol, 1-(1,1-dimethylethyl)amino-3-4-(4-morpholinyl)-1,2,5-thiadiazol-3-yloxy-, (2S)-, (2Z)-2-butenedioate (1:1) (salt);mk950;Betim;betime;Tenopt;WP 934
- CBNumber:
- CB3711352
- Molecular Formula:
- C17H28N4O7S
- Molecular Weight:
- 432.49
- MDL Number:
- MFCD00058356
- MOL File:
- 26921-17-5.mol
- MSDS File:
- SDS
| Melting point | 202-203 °C(lit.) |
|---|---|
| alpha | 24405 -12.0° (c = 5 in 1N HCl); D25 -4.2° |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | powder |
| color | white to off-white |
| optical activity | [α]25/D -5.4°, c = 4.9 in 1 M HCl(lit.) |
| Merck | 14,9444 |
| InChIKey | WLRMANUAADYWEA-NWASOUNVSA-N |
| CAS DataBase Reference | 26921-17-5(CAS DataBase Reference) |
| FDA UNII | P8Y54F701R |
| NCI Drug Dictionary | timolol maleate |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H361 | |||||||||
| Precautionary statements | P280-P301+P312+P330 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-63-36/37/38 | |||||||||
| Safety Statements | 36-37/39-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UA8475000 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
(S)-Timolol maleate price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | T6394 | Timolol maleate salt ≥98% (TLC), powder | 26921-17-5 | 1g | $535 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2593 | Timolol Maleate | 26921-17-5 | 500MG | $213 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP1046 | Timolol maleate British Pharmacopoeia (BP) Reference Standard | 26921-17-5 | 500MG | $223 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1667406 | Timolol maleate United States Pharmacopeia (USP) Reference Standard | 26921-17-5 | 200mg | $436 | 2024-03-01 | Buy |
| Cayman Chemical | 13974 | Timolol (maleate) ≥95% | 26921-17-5 | 25mg | $32 | 2024-03-01 | Buy |
(S)-Timolol maleate Chemical Properties,Uses,Production
Description
Timolol is a non-selective β-adrenergic receptor antagonist with log Kd values of -8.27, -9.86, and -6.8 for binding to human β1-, β2-, and β3-adrenoceptors, respectively. It has been reported that only the (S) enantiomer contributes to the β-blocking effects of racemic timolol, but the weakly active (R) isomer maintains a beneficial effect on intraocular pressure without the undesirable side-effect of bronchial constriction caused by non-selective action of (S)-timolol on β1 and β2 receptors. Timolol has been use alone and in fixed combinations with either prostaglandin analogs or carbonic anhydrase inhibitors to reduce intraocular pressure in research models of ocular hypertension and glaucoma.
Chemical Properties
White Solid
Originator
Blocadren,MSD,UK,1974
Uses
Anti hypertensive, Anti-glaucoma
Uses
Antihypertensive; antiarrhythmic (class II); antianginal; antiglaucoma agent.
Uses
betaadrenergic blocker
Definition
ChEBI: The maleic acid salt of the active (S)-enantiomer of timolol, comprising equimolar amounts of (S)-timolol and maleic acid.
Manufacturing Process
Step A: Preparation of 3-tert-Butylamino-2-Oxopropanol - To an aqueous
solution of tert-butylamine (1 mol) at ambient temperature, there is added
slowly and with vigorous stirring 2 mols bromoacetol. The reaction mixture is
allowed to stand at ambient temperature for about 5 hours whereupon it is
made basic by the addition of sodium hydroxide.
The reaction mixture then is extracted with ether, the excess amine is
removed from the ethereal solution under reduced pressure and the ether
then removed by evaporation to give 3-tert-butylamino-2-oxopropanol.
Step B: A solution of the 3-tert-butylamino-2-oxopropanol in a mixture of
pyridine hydrochloride and pyridine is treated with p-toluenesulfonylchloride.
The mixture is stirred for 1/2 hour at 25° to 30°C and then poured into cold water. The solution is treated with potassium carbonate and the pyridine
evaporated in vacuo at a temperature between 55° and 60°C. The aqueous
residue is treated with potassium carbonate and the mixture extracted with
methylene chloride. Evaporation of the dried extract provides 1-
toluenesulfonyloxy-2-oxo-3-tert-butylaminopropane.
Step C: Preparation of 3-Morpholino-4-(3-tert-Butylamino-2-Oxopropoxy)-
1,2,5-Thiadiazole - The 1-toluenesulfonyloxy-2-oxo-3-tert-butylaminopropane,
prepared as described in Step B, (11 mols) is added to 0.80 N methanolic
sodium methoxide (15 ml) at 0°C. The mixture is stirred for 15 minutes at 0°
to 5°C, treated with 3-morpholino-4-hydroxy-1,2,5-thiadiazole (4.29 grams)
and then refluxed for 16 hours. The solvent is evaporated in vacuo and the
residue is treated with excess potassium carbonate to provide 3-morpholino-
4-(3-butylamino-2-oxopropoxy)-1,2,5-thiadiazole.
Step D: Chemical Reduction Preparation of 3-Morpholino-4-(3-tert_x0002_Butylamino-2-Hydroxypropoxy)-1,2,5-Thiadiazole - The 3-morpholino-4-(3-
tert-butylamino-2-oxopropoxy)-1,2,5-thiadiazole (0.01 mol) is dissolved in
isopropanol (10 ml). To the solution is added sodium borohydride in portions
until the initial evolution of heat and gas subsides. The excess sodium
borohydride is destroyed by addition of concentrated hydrochloric acid until
the mixture remains acidic. The precipitate of sodium chloride is removed,
ether is added, and the solution is concentrated to crystallization. The solid
material is removed by filtration and dried thus providing 3-morpholino-4-(3-
tert-butylamino-2-hydroxypropoxy)-1,2,5-thiadiazole, MP 161° to 163°C (as
hydrochloride).
Alternative Step D: Reduction with a Reductate - Sucrose (1 kg) is dissolved
in water (9 liters) in a 20-liter bottle equipped with a gas trap. Baker's yeast
(Saccharomyces cerevisiae, 1 kg) is made into a paste with water (1 liter) and
added to the sucrose solution with stirring. After lively evolution of gas begins
(within 1 to 3 hours), 3-morpholino-4-(3-tert-butylamino-2-oxopropoxy)-
1,2,5-thiadiazole hydrogen maleate [1.35 mols, prepared by reaction of the 3-
morpholino-4-(3-tert-butylamino-2-oxopropoxy)-1,2,5-thiadiazole with an
equimolar quantity of maleic acid in tetrahydrofuran]. The mixture is allowed
to stand until fermentation subsides, after which the bottle is kept in a 32°C
incubator until all fermentation has ended (in approximately 1 to 3 days). The
yeast is filtered off with addition of diatomaceous earth and the filtrate is
evaporated to dryness to give S-3-morpholino-4β-tert-butylamino-2-
hydroxypropoxy)-1,2,5-thiadiazole, MP 195° to 198°C (as hydrogen maleate),
according to US Patent 3,619,370.
Step E: The base may be converted to the maleate by maleic acid
Therapeutic Function
Antiarrhythmic, Antiglaucoma
Biological Activity
β 1 -adrenergic blocker.
Clinical Use
Beta-adrenoceptor blocker:
Hypertension
Angina
Glaucoma
Migraine prophylaxis
Veterinary Drugs and Treatments
Timolol maleate is used primarily to prevent the development of primary glaucoma in the contralateral eye of a dog which has developed primary glaucoma in one eye. It only reduces intraocular pressure 3 – 10 mmHg and, therefore is of minimal usefulness in patients requiring treatment of primary acute congestive glaucoma. Timolol’s mechanism of action: decreases cyclic-AMP synthesis in non-pigmented ciliary epithelium resulting in decreased aqueous humor production. It may also cause slight miosis in dogs and cats. Timolol maleate is rarely used alone but is combined with dorzolamide solution (Cosopt?). Caution is advised with use of Beta blocking agents in cats with concurrent asthma. As timolol maleate is now available in generic form, it is the primary Beta blocker agent now used.
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: NSAIDs antagonise hypotensive effect.
Anti-arrhythmics: increased risk of myocardial
depression and bradycardia; increased risk of
bradycardia, myocardial depression and AV block
with amiodarone; increased risk of myocardial
depression and bradycardia with flecainide.
Antidepressants: enhanced hypotensive effect with
MAOIs.
Antihypertensives: enhanced hypotensive effect;
increased risk of withdrawal hypertension with
clonidine; increased risk of first dose hypotensive
effect with post-synaptic alpha-blockers such as
prazosin.
Antimalarials: increased risk of bradycardia with
mefloquine.
Antipsychotics: enhanced hypotensive effect with
phenothiazines.
Calcium-channel blockers: increased risk of
bradycardia and AV block with diltiazem;
hypotension and heart failure possible with
nifedipine and nisoldipine; asystole, severe
hypotension and heart failure with verapamil.
Cytotoxics: possible increased risk of bradycardia
with crizotinib.
Diuretics: enhanced hypotensive effect.
Fingolimod: possibly increased risk of bradycardia.
Moxisylyte: possible severe postural hypotension.
Sympathomimetics: severe hypertension with
adrenaline and noradrenaline and possibly with
dobutamine; also response to adrenaline may be
reduced.
Metabolism
Timolol undergoes significant hepatic metabolism, but first pass metabolism is low. The metabolites are excreted in the urine with some unchanged timolol.
(S)-Timolol maleate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 | info@pharmachemm.com | China | 222 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Hebei shuoxi biotechnology co. LTD | +8613081092107 | CHINA | 968 | 58 |
View Lastest Price from (S)-Timolol maleate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-11-12 | (S)-Timolol maleate
26921-17-5
|
US $10.00 / Kg/Bag | 1Kg/Bag | 99% | 20 Tons | Hebei Zhanyao Biotechnology Co. Ltd | |
 |
2021-11-02 | (S)-Timolol maleate
26921-17-5
|
US $206.30-1439.20 / KG | 100g | 99% | 800kg | Baoji Guokang Healthchem co.,ltd | |
 |
2021-10-20 | (S)-Timolol maleate
26921-17-5
|
US $50.00 / KG | 1KG | 99% | 9000kg/per week | Hebei Lingding Biotechnology Co., Ltd. |
-

- (S)-Timolol maleate
26921-17-5
- US $10.00 / Kg/Bag
- 99%
- Hebei Zhanyao Biotechnology Co. Ltd
-

- (S)-Timolol maleate
26921-17-5
- US $206.30-1439.20 / KG
- 99%
- Baoji Guokang Healthchem co.,ltd
-

- (S)-Timolol maleate
26921-17-5
- US $50.00 / KG
- 99%
- Hebei Lingding Biotechnology Co., Ltd.