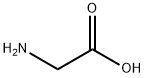
Glycine
- CAS No.
- 56-40-6
- Chemical Name:
- Glycine
- Synonyms
- Gly;H-GLY-OH;Gly-OH;Aminoacetic acid;L-Gly;BLOTTING BUFFER;TGC;GLYCINE USP;Glycine Amino acid;TT BUFFER
- CBNumber:
- CB5336487
- Molecular Formula:
- C2H5NO2
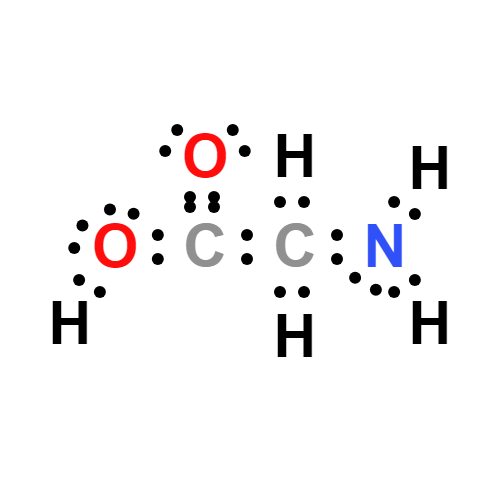
Lewis structure
- Molecular Weight:
- 75.07
- MDL Number:
- MFCD00008131
- MOL File:
- 56-40-6.mol
- MSDS File:
- SDS
| Melting point | 240 °C (dec.) (lit.) |
|---|---|
| Boiling point | 233°C |
| Density | 1.595 |
| vapor pressure | 0.0000171 Pa (25 °C) |
| refractive index | 1.4264 (estimate) |
| FEMA | 3287 | GLYCINE |
| Flash point | 176.67°C |
| storage temp. | 2-8°C |
| solubility | H2O: 100 mg/mL |
| form | powder |
| pka | 2.35(at 25℃) |
| color | <5 (200 mg/mL)(APHA) |
| PH | 4(0.2 molar aqueous solution) |
| Odor | Odorless |
| PH Range | 4 |
| Odor Type | odorless |
| Water Solubility | 25 g/100 mL (25 ºC) |
| λmax |
λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| JECFA Number | 1421 |
| Merck | 14,4491 |
| BRN | 635782 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | DHMQDGOQFOQNFH-UHFFFAOYSA-N |
| LogP | -3.21 |
| FDA 21 CFR | 172.812; 582.5049; 172.320; 310.545 |
| Substances Added to Food (formerly EAFUS) | GLYCINE |
| CAS DataBase Reference | 56-40-6(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | TE7660XO1C |
| NCI Drug Dictionary | glycine |
| ATC code | B05CX03 |
| NIST Chemistry Reference | Glycine(56-40-6) |
| EPA Substance Registry System | Glycine (56-40-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Risk Statements | 33 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | MB7600000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29224910 | |||||||||
| Toxicity | LD50 orally in Rabbit: 7930 mg/kg | |||||||||
| NFPA 704 |
|
Glycine price More Price(158)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | GE17-1323-01 | Glycine Cytiva 17-1323-01, pack of 500?g | 1PKG | $81.6 | 2024-03-01 | Buy | |
| Sigma-Aldrich | 8.16013 | Glycine for synthesis | 56-40-6 | 250g | $70.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.16013 | Glycine for synthesis | 56-40-6 | 1kg | $221 | 2024-03-01 | Buy |
| Sigma-Aldrich | 4810-OP | OmniPurGlycine-CAS56-40-6-Calbiochem | 56-40-6 | 500g | $65.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 4810-OP | OmniPurGlycine-CAS56-40-6-Calbiochem | 56-40-6 | 5kg | $370 | 2024-03-01 | Buy |
Glycine Chemical Properties,Uses,Production
Amino acids with the simplest structure
Glycine is of the simplest structure in the 20 members of amino acid series, also known as amino acetate. It is a non-essential amino acid for the human body and contains both acidic and basic functional group inside its molecule. It exhibits as a strong electrolyte an aqueous solution, and has a large solubility in strong polar solvents but almost insoluble in non-polar solvents. Moreover, it also has a relative high melting point and boiling point. The adjustment of the pH of the aqueous solution can make glycine exhibit different molecular forms. The side chain of glycine contains only a hydrogen atom. Owing to another hydrogen atom connecting to the α-carbon atom, the glycine is not optical isomer. Since the side bond of glycine is very small, it can occupy space which can’t be occupied by other amino acids, such as those amino acids located within the collagen helix. At room temperature, it exhibits as white crystal or light yellow crystalline powder and has a unique sweet taste which can ease the taste of acid and alkaline taste, masking the bitter taste of saccharin in food and enhance the sweetness. However, if an excessive amount of glycine is absorbed by body, they not only can’t be totally absorbed by the body, but will also break the balance of the body's absorption of amino acids as well as affect the absorption of other kinds of amino acids, leading to nutrient imbalances and negatively affected health. The milk drink with glycine being the major raw material can easily does harm to the normal growth and development of young people and children. It has a density of 1.1607, melting point of 232~236 °C (decomposition). It is soluble in water but insoluble in alcohol and ether. It is capable of acting together with hydrochloric acid to form hydrochloride salt. It is presented in the muscles of animals. IT can be produced from the reaction between monochloro acetate and ammonium hydroxide as well as from the hydrolysis of gelation with further refining.
History of discovery
Amino acids are organic acids containing an amino group and are the basic units of protein. They are generally colorless crystals with a relative high melting point (over 200 °C). It is soluble in water with amphiprotic ionization characteristics and can have sensitive colorimetric reaction with ninhydrin reagent. In 1820, glycine with the simplest structure was first discovered in a protein hydrolysis product. Until 1940, it has been found that there were about 20 kinds of amino acids in nature. They are necessary for the protein synthesis of both human and animal. They are mostly α-L-type amino acids. According to the different number of amino groups and carboxyl groups contained in amino acids, we classify amino acids into neutral amino acids (glycine, alanine, leucine, isoleucine, valine, cystine, cysteine, A methionine, threonine, serine, phenylalanine, tyrosine, tryptophan, proline and hydroxyproline, etc.) with the amino acid molecules containing only one amino group and a carboxyl group; acidic amino acid (glutamate, aspartate) which contains two carboxyl and one amino group; alkaline amino acids (lysine, arginine) which molecularly contains one carboxyl group and two amino groups; Histidine contains a nitrogen ring which exhibits weakly alkaline and thus also belonging to alkaline amino acids. Amino acids can be obtained both from protein hydrolysis and from chemical synthesis. Since the 1960s, industrial production mainly applied microbial fermentation, such as monosodium glutamate factory has been widely applied fermentation method for production of glutamate. In recent years, people has also applied petroleum hydrocarbons and other chemical products as raw materials of fermentation for production of amino acids.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Content Analysis
Accurately weigh 175 mg of sample which has undergone drying for 2 h at 105 °C and place it in a 250m1 flask, add 50 mL of glacial acetic acid for dissolving; add 2 drops of crystal violet test solution (TS-74); titrate with 0.1ml/L perchloric acid to blue-green endpoint. At the same time carry out a blank test, and make the necessary corrections. Each mL of 0.1mol/L perchloric acid is equivalent to glycine (C2H5NO2) 7.507mg.
Biosynthesis of glycine production
In the late 1980s, Japan's Mitsubishi Corporation added the screened aerobic Agrobacterium, Brevibacterium, Corynebacterium genus to the medium containing carbon, nitrogen and inorganic nutrient solution for cultivation, and then applied this class of bacteria for converting ethanolamine to glycine in 25~45 °C and pH value from 4 to 9 and further applied concentration, neutralization ion exchange treatment to get the glycine product.
After entering the 1990s, there had been new progress on the production technology of glycine in foreign countries. The Nitto Chemical Industry Co (Japan) add cultured pseudomonas genus, casein bacteria genus, and alcaligenes genus and other species in 0.5% (mass fraction, dry weight) to the glycine amine-containing matrix for reaction of 45 h under 30 °C and pH value of 7.9 to 8.1 with almost all glycine amine being hydrolyzed into glycine with the conversion rate of 99%. Although biological methods are still in the research stage, however, owing to its high selectivity, non-pollution property, it will be a synthetic route with highly development potential.
Uses
- Used for the pharmaceutical industry, organic synthesis and biochemical analysis.
- Used as a buffer for the preparation of tissue culture media and the testing of copper, gold and silver. In medicine, it is used for the treatment of myasthenia gravis and progressive muscular atrophy, hyperacidity, chronic enteritis, and children hyperprolinemia diseases.
- Used for the treatment of myasthenia gravis and progressive muscular atrophy; treatment of excess stomach acid ester disease, chronic enteritis (often in combination antacid); using in combination with aspirin can reduce the irritation of the stomach; treatment of children hyperprolinemia; as the nitrogen source for generating non-essential amino acid and can be added to a mixed amino acid injection.
- Glycine is primarily used as a nutritional additive in chicken feed.
- Used as a kind of nutritional supplement which is mainly used for flavoring.
- Flavoring agent: Used for alcoholic beverage in combination with alanine; the addition amount: grape wine: 0.4%, whiskey: 0.2%, champagne: 1.0%. Others such as powder soup: 2%; lees marinated foods: 1%. Because it is tasted like shrimp and cuttlefish, and thus can be used in sauces.
- It has some certain inhibitory effects on the Bacillus subtilis and E. coli and thus can be used as the preservatives of surimi products and peanut butter with the added amount being 1% to 2%.
- Buffering effect: Because glycine is amphiprotic ions containing both amino and carboxyl groups, it has a strong buffering property on the taste feeling of salt and vinegar. The added amount is: salted products: 0.3% to 0.7%, acid stain product: 0.05% to 0.5%. Antioxidant effect (with its metal chelation): being added to butter, cheese, and margarine extend the storage duration by 3 to 4 times. To make the lard oil in baked food be stable, we can add 2.5% glucose and 0.5% glycine. Adding 0.1% to 0.5% glycine to the wheat flour for making convenient noodles can play a role of flavoring. In pharmacy, it is used as antacids (hyperacidity), therapeutic agent for muscle nutritional disorder as well as antidotes. Moreover, glycine can also be used as the raw material for synthesizing amino acids like threonine.
- It can be used as a spice according to the provisions of GB 2760-96.
- Glycine is also known as aminoacetic acid. In the field of pesticide production, it is used for synthesizing the glycine ethyl ester hydrochloride which is the intermediate for the synthesis of pyrethroid insecticides. Moreover, it can also be used for synthesizing fungicides iprodione and solid glyphosate herbicide; in addition it is also used in various kinds of other industries such as fertilizer, medicine, food additives, and spices.
- Used as a solvent to remove carbon dioxide in the fertilizer industry. In the pharmaceutical industry, it can be used as amino acid preparations, the buffer of chlortetracycline buffer and as the raw material for synthesizing the anti-Parkinson's disease drugs L-dopa. Moreover, it is also the intermediate for producing ethyl imidazole. It is also an adjunct therapy medicine for treating neural hyperacidity and effectively suppressing excess amount of gastric ulcer acid. In the food industry, it is used for the synthesis of alcohol, brewing products, meat processing and cold drinks formula. As a food additive, glycine can be used alone as a condiment and also used in combination with sodium glutamate, DL-alanine acid, and citric acid. In other industries, it can be used as a pH adjusting agent, being added to the plating solution, or used as the raw material for making other amino acids. It can further be used as biochemical reagents and solvent in organic synthesis and biochemistry.
- Used as the intermediates of pharmaceutical and pesticide, decarbonation solvents of fertilizers, plating fluid, etc.
- Used as a solvent for removing carbon dioxide in the fertilizer industry. In pharmaceutical industry, it is used as the buffer of chlortetracycline, amino antacids, and used for the preparation of L-dopa. In food industry, it can be used as flavoring agents, agent for removing saccharine bitter taste, for brewing, meat processing, and preparation of soft drinks. In addition, it can also be used as a pH adjusting agent and used in the preparation of the plating solution.
- Used as biochemical reagents for the pharmaceutical, food and feed additives; it can also be used as a non-toxic decarbonization agent in the field of fertilizer industry.
Description
Glycine (abbreviated as Gly or G) is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its side-chain, glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG of the genetic code.
Glycine is a colourless, sweet-tasting crystalline solid. It is unique among the proteinogenic amino acids in that it is not chiral. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. Glycine is also the genus name of the Soybean plant (species name = Glycine max).
Chemical Properties
A white, odorless, crystalline powder having a sweetish taste. Its solution is acid to litmus. One g dissolves in about 4 mL of water. It is very slightly soluble in alcohol and in ether. Glycine may be prepared from chloroacetic acid and ammonia; from protein sources, such as gelatin and silk fibroin; from ammonium bicarbonate and sodium cyanide; by catalytic cleavage of serine; from hydrobromic acid and methyleneaminoacetonitrile.
Chemical Properties
Glycine is odorless and has a slightly sweet taste.
Chemical Properties
Glycine occurs as a white, odorless, crystalline powder, and has a sweet taste.
Occurrence
Gelatin and silk fbroin are reportedly the best natural sources of this amino acid
Uses
Glycine is a nonessential amino acid that functions as a nutrient and dietary supplement. it has a solubility of 1 g in 4 ml of water and is abundant in collagen. it is used to mask the bitter aftertaste of sac- charin, for example, in artificially sweetened soft drinks. it retards rancidity in fat.
Uses
glycine is an amino acid used as a texturizer in cosmetic formulations. It makes up approximately 30 percent of the collagen molecule.
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. Most glycine is manufactured as USP grade material for diverse uses. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine.
Pharmaceutical grade glycine is produced for some pharmaceutical applications, such as intravenous injections, where the customer’s purity requirements often exceed the minimum required under the USP grade designation. Pharmaceutical grade glycine is often produced to proprietary specifications and is typically sold at a premium over USP grade glycine.
Technical grade glycine, which may or may not meet USP grade standards, is sold for use in industrial applications; e.g., as an agent in metal complexing and finishing. Technical grade glycine is typically sold at a discount to USP grade glycine.
Animal and human foods
Other markets for USP grade glycine include its use an additive in pet food and animal feed. For humans, glycine is sold as a sweetener/taste enhancer. Certain food supplements and protein drinks contain glycine. Certain drug formulations include glycine to improve gastric absorption of the drug.
Cosmetics and miscellaneous applications
Glycine serves as a buffering agent in antacids, analgesics, antiperspirants, cosmetics, and toiletries.
Many miscellaneous products use glycine or its derivatives, such as the production of rubber sponge products, fertilizers, metal complexants.
Chemical feed stock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicide glyphosate. Glyphosate is a non-selective systemic herbicide used to kill weeds, especially perennials and broadcast or used in the cutstump treatment as a forestry herbicide.
Uses
Non-essential amino acid for human development. An inhibitory neurotransmitter in spinal cord, allosteric regulator of NMDA receptors.
Uses
Glycine is a non-essential amino acid for human development. Glycine is an inhibitory neurotransmitter in spinal cord, allosteric regulator of NMDA receptors.
Definition
ChEBI: The simplest (and the only achiral) proteinogenic amino acid, with a hydrogen atom as its side chain.
Production Methods
Chemical synthesis is the most suitable method of preparation of glycine. Amination of chloroacetic acid and the hydrolysis of aminoacetonitrile are the favored methods of production.
Production Methods
Glycine was discovered in 1820, by Henri Braconnot who boiled gelatin with sulfuric acid.
Glycine is manufactured industrially by treating chloroacetic acid with ammonia :
ClCH2COOH + 2 NH3→H2NCH2COOH + NH4Cl
About 15 million kg are produced annually in this way.
In the USA (by GEO Specialty Chemicals, Inc.) and in Japan (by Shoadenko), glycine is produced via the Strecker amino acid synthesis.
Preparation
From chloroacetic acid and ammonia; from protein sources, such as gelatin and silk fbroin; from ammonium bicarbonate and sodium cyanide; by catalytic cleavage of serine; from hydrobromic acid and methyleneaminoacetonitrile.
Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phospho glycerate. In most organisms, the enzyme Serine hydroxy methyl transferase catalyses this transformation via the cofactor pyridoxal phosphate :
serine + tetra hydro folate → glycine +N5,N10-Methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible : CO2 + NH4+ + N5,N10-Methylene tetra hydro folate + NADH + H+→ Glycine + tetrahydrofolate +NAD+
Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35 % glycine.
Biotechnological Production
Glycine is manufactured exclusively by chemical synthesis, and two main processes
are practiced today. The direct amination of chloroacetic acid
with a large excess of ammonia gives good yields of glycine without producing
large amounts of di- and trialkylated products. This process is widely used in
China, where the main application of the glycine is as a raw material for the
herbicide glyphosate.
The other main process is the Strecker synthesis. The direct Strecker reaction of
formaldehyde and ammonium cyanide produces methylene amino acetonitrile,
which must be hydrolyzed in two stages to produce glycine . A more efficient
approach is to aminate the intermediate glycolonitrile, followed by hydrolysis].
An alternative method, which is more often applied for the homologous amino
acids, is the Bucherer–Bergs reaction. Reaction of formaldehyde and ammonium
carbonate or bicarbonate gives the intermediate hydantoin, which can be hydrolyzed
to glycine in a separate step.
Biological Functions
Glycine is another inhibitory CNS neurotransmitter. Whereas GABA is located primarily in the brain, glycine is found predominantly in the ventral horn of the spinal cord. Relatively few drugs are known to interact with glycine; the best-known example is the convulsant agent strychnine, which appears to be a relatively specific antagonist of glycine.
Biological Functions
The principal function of glycine is as a precursor to proteins. It is also a building block to numerous natural products.
As a biosynthetic intermediate
In higher eukaryotes, D-Aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA. Glycine provides the central C2N subunit of all purines.
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potentia (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required coagonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg / kg in rats (oral), and it usually causes death by hyperexcitability. .
General Description
White crystals.
Air & Water Reactions
Water soluble.
Reactivity Profile
An amino acid. A 0.2M aqueous solution has a pH of 4.0., so acts as a weak acid. Has characteristics of both acid and base.
Hazard
Use in fats restricted to 0.01%.
Fire Hazard
LOW. Ignites at very high temperatures.
Pharmaceutical Applications
Glycine is routinely used as a cofreeze-dried excipient in protein
formulations owing to its ability to form a strong, porous, and
elegant cake structure in the final lyophilized product. It is one
of the most frequently utilized excipients in freeze-dried injectable
formulations owing to its advantageous freeze-drying properties.
Glycine has been investigated as a disintegration accelerant in
fast-disintegrating formulations owing to its excellent wetting
nature.It is also used as a buffering agent and conditioner in
cosmetics.
Glycine may be used along with antacids in the treatment of
gastric hyperacidity, and it may also be included in aspirin
preparations to aid the reduction of gastric irritation.
Agricultural Uses
Glycine is the simplest naturally occurring amino acid and is a constituent of most proteins. Its formula is H2N·CH2·COOH.
Biological Activity
One of the major inhibitory neurotransmitters in the mammalian CNS, predominantly active in the spinal cord and brain stem. Also acts as a modulator of excitatory amino acid transmission mediated by NMDA receptors. Also available as part of the NMDA Receptor - Glycine Site Tocriset™ .
Biochem/physiol Actions
Glycine has a pivotal role in lowering the plasma lipid levels in diabetic and obese patients by activating the CNS. During brain hypoxia glycine can stabilize the energetics disturbances in brain mitochondria. It also increases the in vitro development of porcine blastocysts when used along with glucose.
Safety Profile
Moderately toxic by intravenous route. Mildly toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Safety
Glycine is used as a sweetener, buffering agent, and dietary
supplement. The pure form of glycine is moderately toxic by the
IV route and mildly toxic by ingestion.
Systemic absorption of glycine irrigation solutions can lead to
disturbances of fluid and electrolyte balance and cardiovascular and
pulmonary disorders.
LD50 (mouse, IP): 4.45 g/kg
LD50 (mouse, IV): 2.37 g/kg
LD50 (mouse, oral): 4.92 g/kg
LD50 (mouse, SC): 5.06 g/kg
LD50 (rat, IV): 2.6 g/kg
LD50 (rat, oral): 7.93 g/kg
LD50 (rat, SC): 5.2 g/kg
storage
Glycine starts to decompose at 233°C. Store in well-closed containers. Glycine irrigation solutions (95–105% glycine) should be stored in single dose containers, preferably type I or type II glass.
Purification Methods
Crystallise glycine from distilled water by dissolving at 90-95o, filtering, cooling to about -5o, and draining the crystals centrifugally. Alternatively, crystallise it from distilled water by addition of MeOH or EtOH (e.g. 50g dissolved in 100mL of warm water, and 400mL of MeOH is added). The crystals are washed with MeOH or EtOH, then with diethyl ether. Likely impurities are ammonium glycinate, iminodiacetic acid, nitrilotriacetic acid or/and ammonium chloride. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1955 1961, Beilstein 4 IV 2349.]
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants involves the glycine cleavage enzyme Glycine + tetra hydro folate + NAD+ → CO2 + NH4+ + N5,N10-Methylene tetra hydrofolate + NADH + H+ In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase. In the third pathway of glycine degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction. The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life was between 0.5 and 4.0 hours.
Presence in space
The detection of glycine in the interstellar medium has been debated . In 2008, the glycine - like molecule amino aceto nitrile was discovered in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius by the Max Planck Institute for Radio Astronomy . In 2009, glycine sampled in 2004 from comet Wild 2 by the NASA spacecraft Stardust was confirmed, the first discovery of extraterrestrial glycine. That mission's results bolstered the theory of panspermia, which claims that the "seeds" of life are widespread throughout the universe.
Incompatibilities
Glycine may undergo Maillard reactions with amino acids to produce yellowing or browning. Reducing sugars will also interact with secondary amines to form an imine, but without any accompanying yellow-brown discoloration.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM, IV, SC injections; oral; rectal) and approved for irrigant solutions. Included in parenteral (powders for injection; solutions for injection; vaccines; kits for implant) and nonparenteral (orodispersible tablets/oral lyophilizate; powders for inhalation; powders for oral solution; tablets) formulations licensed in the UK.
Glycine Preparation Products And Raw materials
Raw materials
1of7
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2136 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Maanshan Tiantai Biotechnology Co., Ltd. | +86-0555-8889890 +86-17356584060 | GM@tiantaibio-tech.com | China | 72 | 58 |
| Hebei ZB Gamay Biological Technology Co.,Ltd | +86-031189171450 +86-15632359451 | chem@zbvet.net | China | 222 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Suzhou Sanyi Polymer Chemical Technology Co., Ltd. | +8615571922873 | 15571922873@163.com | China | 106 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
Related articles
- Synthesis and Clinical implications of Glycine
- Glycine is integral to the formation of α-helix in the protein. Glycine acts as an inhibitory neurotransmitter. It also acts a....
- Jun 23,2022
- Glycine:Function and Production
- Glycine, the simplest amino acid, obtainable by hydrolysis of proteins. Sweet-tasting, it was among the earliest amino acids t....
- Aug 3,2021
View Lastest Price from Glycine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Glycine
56-40-6
|
US $3.00 / kg | 1kg | 99% | 2000mt | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-26 | Glycine
56-40-6
|
US $1.50 / kg | 5kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-26 | Glycine
56-40-6
|
US $11.00-9.00 / kg | 1kg | 99% | 100Tons | Hebei Dangtong Import and export Co LTD |