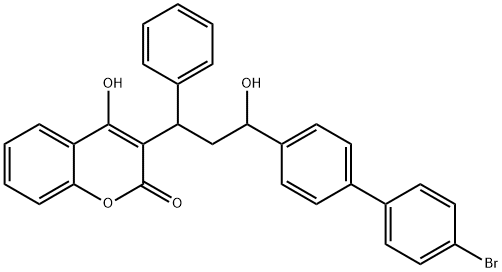
Bromadiolone
- CAS No.
- 28772-56-7
- Chemical Name:
- Bromadiolone
- Synonyms
- BROPRODIFACOUM;MAKI;Miki;Musal;Roban;boldo;lm637;rafix;temus;eradic
- CBNumber:
- CB3756243
- Molecular Formula:
- C30H23BrO4
- Molecular Weight:
- 527.41
- MDL Number:
- MFCD00128053
- MOL File:
- 28772-56-7.mol
| Melting point | 200-210° |
|---|---|
| Boiling point | 723.4±60.0 °C(Predicted) |
| Density | 1.3243 (rough estimate) |
| vapor pressure | 2 x l0-6Pa (20 °C) |
| refractive index | 1.4490 (estimate) |
| storage temp. | -20°C |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; DMSO:PBS (pH 7.2) (1:3): 0.25 mg/ml |
| Water Solubility | 19 mg l-1 (20 °C) |
| form | Powder |
| pka | 4.04(at 21℃) |
| Merck | 13,1366 |
| BRN | 1335336 |
| LogP | 7.480 (est) |
| CAS DataBase Reference | 28772-56-7(CAS DataBase Reference) |
| FDA UNII | J2FR050NM5 |
| Pesticides Freedom of Information Act (FOIA) | Bromadiolone |
| EPA Substance Registry System | Bromadiolone (28772-56-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H360D-H372-H410 | |||||||||
| Precautionary statements | P202-P264-P273-P280-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 27/28-50 | |||||||||
| Safety Statements | 28-36/37/39-45-61 | |||||||||
| RIDADR | UN 2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GN4934700 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | LD50 in rats, mice (mg/kg): 1.125, 1.75 orally (Grand) | |||||||||
| NFPA 704 |
|
Bromadiolone Chemical Properties,Uses,Production
Description
Bromadiolone is used to control rodents around buildings, inside transport vehicles and sewers. It is often formulated as meal bait, rat and mouse bait ready-to-use place packs, paraffinized pellets and blocks.
Chemical Properties
Bromadiolone is white to off-white (yellowish) powder.
Occurrence
Boldo is an evergreen found in Chile, Peru, and Morocco.
Uses
Bromadiolone is used for the control of rats and mice.
Uses
Rodenticide.
Uses
Bromadiolone is an anticoagulant rodenticide used on non-crop areas.
Definition
ChEBI: Bromadiolone is a diarylheptanoid.
General Description
Yellowish powder. Used as an anticoagulant rodenticide.
Health Hazard
The compound is toxic by oral exposure.
Fire Hazard
(Non-Specific -- Coumarin Derivative Pesticide, Solid, n.o.s.) Fire may produce irritating or poisonous gases. Runoff from fire control water may give off poisonous gases. Runoff from fire control or dilution water may cause pollution. When heated to decomposition, Bromadiolone emits toxic fumes of bromine containing compounds.
Agricultural Uses
Rodenticide: Bromadiolone is used as an anticoagulant rodenticide and used as bait for rodent control against house mice, roof rats and warfarin-resistant Norway rats. It is also authorized by USDA for use in official establishments operating under the Federal meat, poultry, shell egg grading and egg products inspection program.
Trade name
BOLDO®; BOOT HILL®; BROMONE®; CANADIEN 2000®; CONTRAC®; HAWK®; LM- 637®; MAKI®; RAT ARREST®; RAT FREE®; RATIMUS®; RENTOKIL DEADLINE®; SLAYMOR®; SUPER-CAID®; SUPER-ROZOL®; SUP'ORATS®; TERMUS®
Potential Exposure
Bromadiolone is used as an anticoagulant rodenticide. It is bait for rodent control used against house mice, roof rats, warfarin-resistant Norway rats. It is also authorized by USDA for use in official establishments operating under the federal meat, poultry, shell egg grading, and egg products inspection program. May be used as a drug.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Keepvictim quiet and maintain normal body temperature. Effectsmay be delayed; keep victim under observation.
Environmental Fate
Bromadiolone belongs to second generation of long-acting anticoagulant rodenticide. It acts by interfering with the prothrombin synthesis by blocking the regeneration of vitamin K dependant proteins in the liver and thereby disrupting the clotting mechanisms and increasing the tendency to hemorrhages and subsequent death.
Metabolic pathway
Metabolism in the rat is very slow and products other than a conjugate of the parent compound have not been identified. Biodegradation in soil and plants has not been reported.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with bromadiolone you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area.
Shipping
UN3027 Coumarin derivative pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1—Poisonous materials. UN3026 Coumarin derivative pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Toxicity evaluation
Bromadiolone may be released into the environment
through various waste streams and its use.
If released to air, it will exist in the particulate phase in the
ambient atmosphere and will be removed by dry and wet deposition. In soil, it is not persistent under aerobic conditions
(half-life is 14 days) and is usually immobile except in soils of
low organic matter and clay, such as sand.
When released to water, it adsorbs to suspended solids
and sediment. Bromadiolone is stable to hydrolysis at pH 5,
7, and 9. Two major degradates, [1,3-diphenyl-5(40-bromobiphenyl)
pentane-1-ol] and [1,3-diphenyl-5(40-bromobiphenyl)
pentane-1,5-diol], are detected in the aerobic soil
metabolism study.
Bromadiolone is bioaccumulated in edible and nonedible
tissues in bluegill sunfish at the bioaccumulation concentration
factors of 160X and 1658X. It was also detected in birds.
Degradation
Bromadiolone is a stable compound. It is a weak acid.
Bromadiolone Preparation Products And Raw materials
Raw materials
1of3