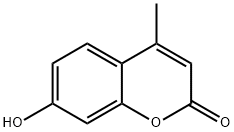
4-Methylumbelliferone
- CAS No.
- 90-33-5
- Chemical Name:
- 4-Methylumbelliferone
- Synonyms
- HYMECROMONE;7-HYDROXY-4-METHYL-2H-CHROMEN-2-ONE;7-HYDROXY-4-METHYLCOUMARIN;4-MU;4-Methylumbelliferon;4-METHYL-7-HYDROXYCOUMARIN;7-HYDROXY-4-METHYL-CHROMEN-2-ONE;Medilla;Bilcolic;Hymecromon
- CBNumber:
- CB5745737
- Molecular Formula:
- C10H8O3
- Molecular Weight:
- 176.17
- MDL Number:
- MFCD00006866
- MOL File:
- 90-33-5.mol
| Melting point | 188.5-190 °C(lit.) |
|---|---|
| Boiling point | 267.77°C (rough estimate) |
| Density | 1.1958 (rough estimate) |
| refractive index | 1.5036 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water; slightly soluble in ethanol, ether,chloroform; soluble inmethanol |
| form | Crystalline Powder |
| pka | 7.79(at 25℃) |
| color | off-white to yellow |
| PH Range | Green 1 uorescent (0.0) to weak blue 1 uorescent (2.0);Weak blue 1 uorescent (6.5) blue 1 uorescent (8.0) |
| Water Solubility | slightly soluble |
| λmax | 221nm, 251nm, 322nm |
| Merck | 14,4854 |
| BRN | 142217 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Major Application | color filter, electroluminescent displays, document security processes, lasers, integrated circuits, liquid crystal displays, surface brightening composition, oil products, teeth whitening agent, lysosomal enzyme assay, substrate for lipase, determining protein-protein-interactions in living cells, Antitrypanosomal, antileishmanial, anticancer, antiHIV, antimalarial, antibacterial, antifungal |
| InChIKey | HSHNITRMYYLLCV-UHFFFAOYSA-N |
| LogP | 2.370 (est) |
| CAS DataBase Reference | 90-33-5(CAS DataBase Reference) |
| EWG's Food Scores | 4-7 |
| FDA UNII | 3T5NG4Q468 |
| ATC code | A05AX02 |
| NIST Chemistry Reference | Coumarin, 7-hydroxy-4-methyl-(90-33-5) |
| EPA Substance Registry System | 4-Methylumbelliferone (90-33-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H303-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-37/39-36 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GN7000000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29329990 | |||||||||
| Toxicity | LD50 oral in rat: 3850mg/kg | |||||||||
| NFPA 704 |
|
4-Methylumbelliferone price More Price(46)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M1381 | 4-Methylumbelliferone ≥98% | 90-33-5 | 25g | $36.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | M1381 | 4-Methylumbelliferone ≥98% | 90-33-5 | 100g | $69.7 | 2024-03-01 | Buy |
| TCI Chemical | M0453 | 4-Methylumbelliferone >98.0%(T) | 90-33-5 | 25g | $108 | 2024-03-01 | Buy |
| TCI Chemical | M0453 | 4-Methylumbelliferone >98.0%(T) | 90-33-5 | 100g | $266 | 2024-03-01 | Buy |
| Alfa Aesar | A10337 | 4-Methylumbelliferone, 97% | 90-33-5 | 50g | $36.6 | 2024-03-01 | Buy |
4-Methylumbelliferone Chemical Properties,Uses,Production
Chemical Properties
Off-white to yellow crystals. Soluble in ethanol, acetic acid, alkali solutions, and ammonia. Slightly soluble in hot water, ether, and chloroform. Exhibits blue fluorescence when exposed to concentrated sulfuric acid.
Originator
Odeston,Merck
Uses
4-methylumbelliferone (4-MU) is a dietary supplement that inhibits hyaluronic acid (HA) synthesis. HA is a prominent component of the extracellular matrix at many sites of chronic inflammation, including type 1 diabetes (T1D), multiple sclerosis, and numerous malignancies. 4-MU is an already approved drug in Europe and Asia called “hymecromone” where it is used to treat biliary spasm.
Definition
ChEBI: 4-methylumbelliferone is a hydroxycoumarin that is umbelliferone substituted by a methyl group at position 4. It has a role as an antineoplastic agent and a hyaluronic acid synthesis inhibitor. It is functionally related to an umbelliferone.
Preparation
4-Methylumbelliferone is obtained by the cyclization reaction between resorcinol and ethyl acetate. Concentrated sulfuric acid is cooled to below 10 ° C, and a solution prepared from resorcinol and ethyl acetate is slowly added to the stirring solution. After dropping it completely, let it stand for 12 hours. Then, the reaction mixture is added into ice-cold water. The precipitate is filtered out and purified with a dilute sodium hydroxide solution and dilute sulfuric acid to obtain the final product.
Manufacturing Process
Resorcin reacted with 3-oxo-butyric acid ethyl ester in the presence sulfuric acid and phosphorous pentaoxide and 4-methyl-7-hydroxycoumarine (hymecromone) was obtained.
Therapeutic Function
Choleretic, Spasmolytic, Sunscreen agent
General Description
4-Methylumbelliferone is a useful indicator for nitric acid determination and enzyme activity investigation. It emits fluorescence in alkaline solution and Standard for the fluorometric determination of enzyme activity. Highly fluorescent in alkaline solution. Fluorescence: max Abs. l = 360nm, max. Em. l = 449nm; pH > 9.
Air & Water Reactions
Insoluble in water.
Fire Hazard
Flash point data for 4-Methylumbelliferone are not available. 4-Methylumbelliferone is probably combustible.
Pharmaceutical Applications
4-Methylumbelliferone is primarily used in synthesizing medicinal compounds and as a building block for fluorescent probes. Applications include synthesis of:
coumarin triazole derivatives as potential antimicrobial agents.
coumarin salen-based fluorescence sensors for Mg2+ detection.
pyranocoumarin derivatives as anti-hyperglycemic and anti-dyslipidemic agents.
coumarin piperazine derivatives as potential multireceptor atypical antipsychotics.
4-methylumbelliferyl T-antigen as a substrate for endo-α-N-acetylgalactosaminidase.
Biological Activity
4-methylumbelliferone is a hyaluronic acid (ha) synthesis inhibitor. the activation of has2 and the over-production of ha are existed in many metastatic tumor cell lines. increased synthesis of ha is often associated with increased metastatic potential and invasivity in tumor cells.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
in vitro
4-methylumbelliferone (mu), an inhibitor of ha synthesis, has been studied as a potential anti-tumor drug on account of inhibiting the growth of primary tumors and distant metastasis of tumor cells. the mechanism still needs to be clarified, although several studies revealed that the anticancer effects of mu are mediated by inhibition of ha signal pathway. in a previous study the regulation of ha synthesis was demonstrated by ceramide, and now show how mu activated nsmase2 generates ceramides and mediates mu modulated inhibition of ha synthesis (cell migration and invasion, and apoptosis of tumor cells). using a ha enriched mouse oligodendroglioma cell line g26-24, it was found that mu elevated the activity of nsmase2 and enhanced ceramide levels, which in turn potentiated phosphatase pp2a activity. further, the activated pp2a decreased phosphorylation of akt, reduced activities of ha synthase2 (has2) and calpains, and blocked both the synthesis of ha, and the migration and invasion of g26-24 tumor cells. in addition, mu mediated ceramide induced activation of p53 and caspase-3, decreased sirt1 expression and deduced g26-24 viability. the mechanism of the mu anticancer therefore initially involves nsmase2/ceramide/pp2a/akt/has2/caspase-3/p53/sirt1 and the calpain signaling pathway, indicating that ceramides play a important role in the ability of a tumor to become aggressively metastatic and grow [1].
target
P450 (e.g. CYP17) | NADPH-oxidase | PKC | ROS
IC 50
0.4 mm
Purification Methods
Purify it by recrystallisation from EtOH. It is very slightly soluble in cold H2O (solubility at 37o is 0.22%), slightly soluble in Et2O and CHCl3, but soluble in MeOH and AcOH. It has a blue fluorescence in aqueous EtOH and has UV: 221, max 251 and 322.5nm (MeOH). The IR has 3077 br, 1667, 1592, 1385, 1267, 1156, 1130 and 1066 cm-1.max The acetate has m 153-154o. [Woods & Sapp J Org Chem 27 3703 1962, Beilstein 18 III/IV 332, 18/1 V 439.]
References
[1] qin j, kilkus j, dawson g. the hyaluronic acid inhibitor 4-methylumbelliferone is an nsmase2 activator-role of ceramide in mu anti-tumor activity. biochim biophys acta. 2016 feb;1861(2):78-90.
[2] Mustafa, Yasser Fakri et al. “4-Methylumbelliferone And Its Derived Compounds: A Brief Review Of Their Cytotoxicity.” The Egyptian Journal of Chemistry (2021): 0-0.
[3] Essa, Marwa Labib et al. “Dual targeting nanoparticles based on hyaluronic and folic acids as a promising delivery system of the encapsulated 4-Methylumbelliferone (4-MU) against invasiveness of lung cancer in vivo and in vitro.” International journal of biological macromolecules (2022): n. pag.
4-Methylumbelliferone Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 2259 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Rixing Chemical CO.,LTD | +86-13237129059 -13237129059 | sales@rixingchemi.com | CHINA | 229 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
View Lastest Price from 4-Methylumbelliferone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-23 | 4-Methylumbelliferone
90-33-5
|
US $15.00 / kg | 1kg | 99.912% | 10ton | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-09 | 4-Methylumbelliferone
90-33-5
|
US $0.00-0.00 / Kg | 1Kg | 99.9% | 200tons | airuikechemical co., ltd. | |
 |
2024-03-27 | 4-Methylumbelliferone
90-33-5
|
US $0.00-0.00 / kg | 1kg | 99% | 50000kg | Hebei Kingfiner Technology Development Co. , Ltd. |
-

- 4-Methylumbelliferone
90-33-5
- US $15.00 / kg
- 99.912%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- 4-Methylumbelliferone
90-33-5
- US $0.00-0.00 / Kg
- 99.9%
- airuikechemical co., ltd.
-

- 4-Methylumbelliferone
90-33-5
- US $0.00-0.00 / kg
- 99%
- Hebei Kingfiner Technology Development Co. , Ltd.