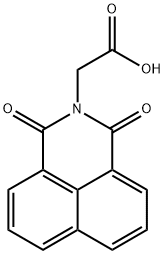
ALRESTATIN
- CAS No.
- 51411-04-2
- Chemical Name:
- ALRESTATIN
- Synonyms
- AY-22284;ALRESTATIN;AURORA 5841;AKOS BBS-00007376;IFLAB-BB F0863-0248;Alrestatin free acid;1,3-dioxo-1h-benz(de)isoquinoline-2(3h)-aceticaci;2-(1,3-dioxobenzo[de]isoquinolin-2-yl)acetic acid;1,3-Dioxo-1H-benzo[de]isoquinoline-2(3H)-acetic acid;1,3-DIOXO-1H-BENZ[D,E]ISOQUINOLINE-2(3H)-ACETIC ACID
- CBNumber:
- CB4392682
- Molecular Formula:
- C14H9NO4
- Molecular Weight:
- 255.23
- MDL Number:
- MFCD00181399
- MOL File:
- 51411-04-2.mol
- MSDS File:
- SDS
| Melting point | 266-267 °C |
|---|---|
| Boiling point | 512.7±33.0 °C(Predicted) |
| Density | 1.511±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO : 50 mg/mL (195.90 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) |
| form | White crystalline solid. |
| pka | 3.61±0.10(Predicted) |
| FDA UNII | 515DHK15LG |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| NFPA 704 |
|
ALRESTATIN price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 29888 | Alrestatin | 51411-04-2 | 5mg | $86 | 2024-03-01 | Buy |
| Cayman Chemical | 29888 | Alrestatin | 51411-04-2 | 50mg | $951 | 2024-03-01 | Buy |
| Cayman Chemical | 29888 | Alrestatin | 51411-04-2 | 10mg | $135 | 2024-03-01 | Buy |
| Cayman Chemical | 29888 | Alrestatin | 51411-04-2 | 25mg | $315 | 2024-03-01 | Buy |
| Tocris | 0485 | Alrestatin | 51411-04-2 | 50 | $411 | 2021-12-16 | Buy |
ALRESTATIN Chemical Properties,Uses,Production
Originator
Alrestatin,BIOMOL
Uses
Enzyme inhibitor (aldose reductase).
Uses
Alrestatin is an inhibitor of aldose reductase and has been shown to display inhibitory activity on aldose reductase prepared and purified from the human brain.
Manufacturing Process
1,3-Dioxo-1H-benz[de]isoquinoline-2(3H)-acetic acid:
1,8-Naphthalic acid anhydride (110 g, 0.556 mole), glycine (48 g, 0.64 mole)
and dimethylformamide (750 ml) are heated and stirred at reflux for 2 hr. The
homogeneous dark solution is cooled to about 100°C and 750 ml of hot water
is added slowly to the stirred solution. The reaction mixture is cooled and
allowed to stand in a refrigerator for 16 hr. The precipitate is collected and
recrystallized from ethanol, using decolorizing charcoal, to give the title
compound, MP: 271°-272°C.
In practice it is usually used as sodium salt.
Therapeutic Function
Aldose reductase inhibitor
Biological Activity
Specific inhibitor of aldose reductase (IC 50 = 148 μ M). Attenuates glucose-induced angiotensin II production in rat vascular smooth muscle in vitro .
Enzyme inhibitor
This aldose reductase inhibitor and drug (FWfree-acid = 255.23 g/mol; CAS 51411-04-2), also known as 1,3-dioxo-1H-benz[de]isoquinoline-2(3H)- acetic acid) suppresses diabetes-associated, osmotic cell and tissue damage by inhibiting aldose reduction and thereby reducing the accumulation intracellular sorbitol. Primary Mode of Action of Aldose Reductase Inhibitors: A major cause of diabetic neuropathy is the intraneural osmotic pressure that builds up as a consequence of the over-accumulation of sorbitol, a polyol formed by aldose reductase (Reaction: Glucose + NADPH ? Sorbitol + NADP+ + H+). Similar considerations apply to cataract formation in the lens, another tissue rich in aldose reductase. In diabetes, aldose reductase activity increases as the concentration of glucose rises in the lens, peripheral nerves and glomerulus (tissues that are insulininsensitive); because sorbitol lacks a membrane carrier, its contributes to intracellular osmotic pressure, disrupting cell-cell interactions (especially synapses), eventually leading to retinopathy and neuropathy. The additive effects of aldose reductase (AR) and polyol dehydrogenase in producing sorbitol from glucose and fructose, acting in combination with agedependent decreased hexokinase is believed to account for diabetic cataract formation in human lenses under high glucose stress. AR’s Km for glucose of AR is roughly 200 mM, whereas its Km for NADPH is 0.06 mM. NADP inhibits human lens AR noncompetitively and has a K1 that is roughly equal to the Km for NADPH. Notably. The Km for fructose is 40 mM and that for NADH is 0.02 mM in the polyol dehydrogenase (PD) reaction. Therefore, although sorbitol formation is modest during normoglycemia, such is not the case for diabetic hyperglycemia. Moreover, the recent increased reliance on high-fructose corn syrup as a sweetener is problematic, in that glucose-sensing mechanisms in humans are largely unresponsive to fructose. Because sorbitol is not transported out of the lens, any increase in intracellular sortbitol must be compensated osmotically by the considerable uptake of water, a well-characterized cataractogenic event. By inhibiting sorbitol dehydrogenase, alrestatin lowers tha undesirable net accumulatrion of sorbitol during hyperglycemic episodes. At high enough concentrations, alrestatin also inhibits PD. Target(s): aldose reductase, or aldehyde reductase; 4-aminobutyrate aminotransferase; carbonyl reductase; succinate-semialdehyde dehydrogenase; polyol dehydrogenase, weakly inhibited, except at elevated concentrations; hexonate dehydrogenase, or glucuronate reductase.
ALRESTATIN Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9635 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10523 | 58 |
| InvivoChem | +1-708-310-1919 +1-13798911105 | sales@invivochem.cn | United States | 6393 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | sales@chemhifuture.com | China | 3136 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| ZHEJIANG JIUZHOU CHEM CO., LTD | +86-0576225566889 +86-13454675544 | admin@jiuzhou-chem.com;jamie@jiuzhou-chem.com;alice@jiuzhou-chem.com | China | 20000 | 58 |
View Lastest Price from ALRESTATIN manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
![2-(1,3-dioxobenzo[de]isoquinolin-2-yl)acetic acid pictures](https://img.chemicalbook.com/ProductImageEN/2019-7/Small/00d89c9a-4b7e-4d37-95d3-090fc5164311.png) |
2019-07-10 | 2-(1,3-dioxobenzo[de]isoquinolin-2-yl)acetic acid
51411-04-2
|
US $8.80 / KG | 1KG | 97%-99% | 100kg | Career Henan Chemical Co |
-
![2-(1,3-dioxobenzo[de]isoquinolin-2-yl)acetic acid pictures](https://img.chemicalbook.com/ProductImageEN/2019-7/Small/00d89c9a-4b7e-4d37-95d3-090fc5164311.png)
- 2-(1,3-dioxobenzo[de]isoquinolin-2-yl)acetic acid
51411-04-2
- US $8.80 / KG
- 97%-99%
- Career Henan Chemical Co