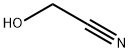
Glycolonitrile
- CAS No.
- 107-16-4
- Chemical Name:
- Glycolonitrile
- Synonyms
- 2-HYDROXYACETONITRILE;HYDROXYACETONITRILE;Acetonitrile, hydroxy-;FORMALDEHYDE CYANOHYDRIN;Glyconitrile;HOCH2CN;usafa-8565;USAF a-8565;Glykolonitril;cyanomethanol
- CBNumber:
- CB4781244
- Molecular Formula:
- C2H3NO
- Molecular Weight:
- 57.05
- MDL Number:
- MFCD00042731
- MOL File:
- 107-16-4.mol
| Melting point | -72°C |
|---|---|
| Boiling point | 183°C |
| Density | 1.076 g/mL at 20 °C |
| refractive index |
n |
| Flash point | 133 °F |
| pka | 11.31±0.10(Predicted) |
| Water Solubility | >=10 g/100 mL at 20 ºC |
| Dielectric constant | 27.0(20℃) |
| Stability | Stable, but may react violently with alkalies. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 107-16-4(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | ZCI474BE63 |
| NIST Chemistry Reference | Acetonitrile, hydroxy-(107-16-4) |
| EPA Substance Registry System | Formaldehyde cyanohydrin (107-16-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310+H330 |
| Precautionary statements | P260-P262-P264-P280-P302+P352+P310-P304+P340+P310 |
| Hazard Codes | T+ |
| Risk Statements | 26/27/28 |
| Safety Statements | 36/37/39-45-36/37-28 |
| RIDADR | UN 3276 6.1/PG 1 |
| WGK Germany | - |
| RTECS | AM0350000 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Toxicity | mouse,LCLo,inhalation,27ppm/8H (27ppm),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE,"Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 290, 1969. |
Glycolonitrile Chemical Properties,Uses,Production
Description
Formaldehyde cyanohydrin is a colorless,odorless oily liquid with a sweetish taste. Molecularweight=57.06; Boiling point=186℃ (slight decomposition); Freezing/Melting point #272℃; Flash point≥93℃. Hazard Identification (based on NFPA-704 MRating System): Health 4, Flammability 2, Reactivity 2.Soluble in water.
Chemical Properties
light yellow liquid (typically available as a concentrated. Glycolonitrile [107-16-4], hydroxyacetonitrile, formaldehyde cyanohydrin, HOCH2CN, Mr 57.05, bp 183℃ (slight decomp.), d154 1.104, n20D1.4117. Anhydrous glycolonitrile is a colorless liquid which is miscible with water, ethanol and diethyl ether.
Chemical Properties
Formaldehyde cyanohydrin is a colorless, odorless, oily liquid. Sweet taste (very highly toxic; do not test).
Uses
Solvent and organic intermediate.
Production Methods
Glycolonitrile is the result of reaction between formaldehyde and aqueous sodium cyanide in the presence of mineral acid.
Definition
ChEBI: Glycolonitrile is an cyanohydrin that is acetonitrile in which one of the hydrogens has been replaced by a hydroxy group.
General Description
Odorless colorless oil with a sweetish taste. Used in the manufacture of intermediates in pharmaceutical production, as a component of synthetic resins, as a chemical intermediate for organic compounds, and as a solvent.
Air & Water Reactions
Water soluble.
Reactivity Profile
Glycolonitrile may undergo spontaneous and violent decomposition. Traces of alkali (base) promote violent polymerization [Lewis].
Hazard
Toxic by ingestion, inhalation, and skin absorption.
Health Hazard
Extremely toxic, exposure by any route should be avoided; may have fatal consequences; death from asphyxiation may occur similar to that resulting from hydrogen cyanide.
Fire Hazard
Moderate explosion hazard when exposed to heat or by spontaneous chemical reaction in the presence of alkalies if uninhibited. When heated to decomposition, Glycolonitrile emits highly toxic fumes of cyanide and nitrogen oxides. Unstable, may explode on standing. Hazardous polymerization may occur. avoid the presence of alkalis, and exposure to heat.
Potential Exposure
Formaldehyde cyanohydrin is used in the manufacture of intermediates in pharmaceutical produc tion and as a component of synthetic resins as a chemical intermediate for organic compounds, and as a solvent.
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 min,occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, removecontaminated clothing and wash immediately with soap andwater. Speed in removing material from skin is of extremeimportance. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescuebreathing (using universal precautions, including resuscitationmask) if breathing has stopped and CPR if heart action hasstopped. Transfer promptly to a medical facility. When thischemical has been swallowed, get medical attention. Givelarge quantities of water and induce vomiting. Do not make anunconscious person vomit. Keep victim quiet and maintainnormal body temperature. Effects may be delayed; keep victimunder observation. Use amyl nitrate capsules if symptomsdevelop. All area employees should be trained regularly inemergency measures for cyanide poisoning and in CPR. Acyanide antidote kit should be kept in the immediate workarea and must be rapidly available. Kit ingredients should bereplaced every 1-2 years to ensure freshness. Persons trainedin the use of this kit, oxygen use, and CPR must be quicklyavailable.
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Store in a refrigerator under an inert atmosphere and awayfrom all alkaline materials. Where possible, automaticallypump liquid from storage containers to process containers.
Shipping
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required, Potential Inhalation Hazard (Special Provision 5). UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and exposure to heat. Unless stabilized with a weak acid solution, traces of alka lis may cause violent polymerization.
Glycolonitrile Preparation Products And Raw materials
Raw materials
Preparation Products
1of2