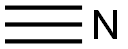HYDROGEN CYANIDE
- CAS No.
- 74-90-8
- Chemical Name:
- HYDROGEN CYANIDE
- Synonyms
- HCN;Hydrocyanic acid;prussic acid;formonitrile;Blausure;methanenitrile;Formic nitrile;HYDROGEN CYANIDE;LELOWRISYMNNSU-UHFFFAOYSA-N;hydrogen cyanide hydrocyanic acid
- CBNumber:
- CB7227244
- Molecular Formula:
- CHN
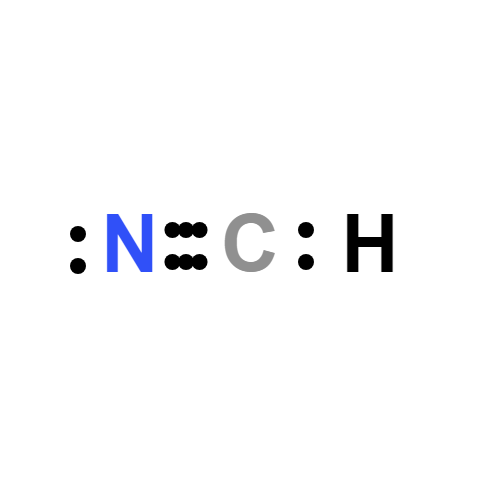
Lewis structure
- Molecular Weight:
- 27.03
- MDL Number:
- MFCD00242847
- MOL File:
- 74-90-8.mol
| Melting point | -13.4° |
|---|---|
| Boiling point | bp 25.6° |
| Density | d(gas) 0.941 (air = 1); d(liq) 0.687 |
| vapor pressure | 750 mmHg at 25 °C |
| refractive index | 1.2594 |
| solubility | very soluble in H2O, ethanol; soluble in ethyl ether |
| form | colorless liquid |
| pka | 9.2(at 25℃) |
| color | colorless liquid or gas |
| Odor | Bitter almond odor detectable at 1 to 5 ppm; however, 20 to 60% of the population are reported to be unable to detect the odor of HCN |
| Water Solubility | miscible with H2O, alcohol; slightly soluble ether [MER06] |
| Exposure limits | Ceiling 11 mg/m3 (10 ppm) (ACGIH), 5 mg CN/m3/10 min (NIOSH); TWA air 11 mg/m3 (10 ppm) skin (OSHA); IDLH 50 ppm. |
| Dielectric constant | 158.0(0℃) |
| LogP | -0.250 |
| EWG's Food Scores | 3-6 |
| NCI Dictionary of Cancer Terms | hydrogen cyanide |
| FDA UNII | 2WTB3V159F |
| Pesticides Freedom of Information Act (FOIA) | Hydrogen cyanide |
| EPA Substance Registry System | Hydrogen cyanide (74-90-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H310-H330-H410 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P262-P264-P270-P280-P302+P350-P310-P322-P361-P363-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P273-P391-P501 | |||||||||
| Hazard Codes | F+,T+,N | |||||||||
| Risk Statements | 12-26-50/53-26/27/28 | |||||||||
| Safety Statements | 7/9-16-36/37-38-45-60-61 | |||||||||
| RIDADR | 1051 | |||||||||
| Autoignition Temperature | 538 °C | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | LC50 in rats, mice, dogs: 544 ppm (5 min), 169 ppm (30 min), 300 ppm (3 min) by inhalation, K. C. Back et al., Reclassification of Materials Listed as Transportation Health Hazards (TSA-20-72-3; PB214-270, 1972) | |||||||||
| IDLA | 50 ppm | |||||||||
| NFPA 704 |
|
HYDROGEN CYANIDE Chemical Properties,Uses,Production
Description
Hydrocyanic acid, HCN, is corrosive in addition to toxic. It is also a dangerous fire and explosion risk. It has a wide flammable range of 6%–41% in air. The boiling point is 79°F (26°C), the flash point is 0°F, and the ignition temperature is 1004°F (540°C). It is toxic by inhalation and ingestion and through skin absorption. The TLV of hydrocyanic acid is 10 ppm in air. It is used in the manufacture of acrylonitrile, acrylates, cyanide salts, dyes, rodenticides, and other pesticides.
Chemical Properties
HCN is a colorless to pale blue liquid or gas. It has a distinct odor resembling bitter almonds. HCN reacts with amines, oxidizers, acids, sodium hydroxide, calcium hydroxide, sodium carbonate, caustic substances, and ammonia. HCN was fi rst isolated from a blue dye, Prussian blue, in 1704. HCN is obtainable from fruits that have a pit, such as cherries, apricots, and bitter almonds, from which almond oil and fl avoring are made. HCN is used in fumigating, electroplating, mining, and in producing synthetic fi bers, plastics, dyes, and pesticides. It is also used as an intermediate in chemical syntheses. Exposures to cyanide occur in workplaces such as the electroplating, metallurgical, fi refi ghting, steel manufacturing, and metal cleaning industries. Human exposures to cyanide also occur from wastewater discharges of industrial organic chemicals, iron and steel works, and wastewater treatment facilities
Chemical Properties
Hydrocyanic acid (hydrogen cyanide) is a clear colorless liquid with a faint odor of bitter almonds. It evaporates easily (or boils) at room temperature and the vapors are slightly lighter than air. It is soluble in water. It is reactive and incompatible with amines, oxi- dizers, acids, sodium hydroxide, calcium hydroxide, sodium carbonate, caustics, and ammonia. Hydrogen cyanide is manufactured by the oxidation of ammonia–methane mixtures under controlled conditions and by the catalytic decomposition of formamide. It may be generated by treating cyanide salts with acid, and it is a combustion by-product of nitrogen-containing materials such as wool, silk, and plastics. It is also produced by enzy- matic hydrolysis of nitriles and related chemicals. Hydrogen cyanide gas is a by-product of coke-oven and blast furnace operations. Industrial applications of hydrogen cyanide are many. For instance, in fumigation, electroplating, mining, metallurgical, fi refi ghting, steel manufacturing, and metal cleaning industries, to producing synthetic fi bers, plastics, dyes, pesticides, and also as an intermediate in chemical syntheses.
Chemical Properties
Water-white liquid at temperatures below 26.5C; faint odor of bitter almonds. Usual commercial material is 96–99% pure.Soluble in water. The solution is weakly acidic, sensitive to light. When not absolutely pure or stabilized, hydrogen cyanide polymerize
Physical properties
Colorless liquid or gas; odor of bitter almond; burns in air with a blue flame;refractive index 1.2675; autoignition temperature 538°C; vapor density at31°C 0.947 (air=1); liquid density 0.715 g/mL at 0°C and 0.688 g/mL at 20°C;boils at 25.7°C; melts at 13.24°C; vapor pressure 264 torr at 0°C; critical tem-perature 183.5°C; critical pressure 53.20 atm; critical volume 139 cm3/moldielectric constant 158.1 at 0°C and 114.9 at 20°C; conductivity 3.3 mhos/cmat 25°C; viscosity 0.201 centipoise at 20°C; surface tension 19.68 dyn/cm;readily mixes with water and alcohols; density of a 10% aqueous solution0.984 g/mL at 20°C; pKaat 25°C 9.21.
Occurrence
Peaches, apricots, bitter almonds, cherries, and plums contain some HCN derivatives in their kernels, frequently in combination with glucose and benzaldehyde as a glucoside (amygdalin). The bitter almond fragrance of HCN and its derivatives sometimes can be detected in such kernels.
History
Hydrogen cyanide in pure form was prepared first in 1815 by Gay-Lussac.Earlier, in 1782, Scheel prepared this compound in dilute solution. The mostimportant application of hydrogen cyanide is to produce methyl methacrylatefor methacrylate resins and plastics. Other products made from hydrogencyanide include potassium cyanide, sodium cyanide, adiponitrile, methionine,cyanuric chloride, cyanogen, nitrilotriacetic acid, and several triazine pesti-cides. The compound also is used in small amounts for extermination ofrodents.
Uses
Hydrogen cyanide is used to produce methyl methacrylate, cyanuric chloride, triazines, sodium cyanide, and chelates such as ethylenediaminetetraacetic acid (EDTA); and in fumigation. It occurs in beet sugar residues and coke oven gas. It occurs in the roots of certain plants, such as sorghum, cassava, and peach tree roots (Adewusi and Akendahunsi 1994; Branson et al. 1969; Esquivel and Maravalhas 1973; Israel et al. 1973) and in trace amounts in apricot seeds (Souty et al. 1970) and tobacco smoke (Rickert et al. 1980). Suchard et al. (1998) have reported a case of acute cyanide poisoning caused by ingestion of apricot kernel. The symptoms were weakness, dyspnea, comatose, and hypothermia observed within 20 minutes of ingestion.
Firefighters chance a great risk to the exposure to HCN, which is a known fireeffluent gas. Materials such as polyurethane foam, silk, wool, polyacrylonitrile, and nylon fibers burn to produce HCN (Sakai and Okukubo 1979; Yamamoto 1979; Morikawa 1988; Levin et al. 1987; Sumi and Tsuchiya 1976) along with CO, acrolein, CO2, formaldehyde, and other gases. Emissions of these toxic gases take place primarily under the conditions of oxygen deficiency, and when the air supply is plentiful the emissions are decreased considerably (Hoschke et al. 1981).
Bertol et al. (1983) determined that 1 g of polyacrylonitrile generated 1500 ppm of HCN. Thus a lethal concentration of HCN could be obtained by burning 2 kg of polyacrylonitrile in an average-sized living room.
Jellinek and Takada (1977) reported evolution of HCN from polyurethanes as a result of oxidative thermal degradation while no HCN evolved from pure thermal degradation. Copper inhibited HCN liberation due to the catalytic oxidation of evolved HCN. Herrington (1979) observed that the isocyanate portion of polyurethane foam volatilizes first, releasing heat, smoke, HCN, nitrogen oxides, and organic compounds. Volatilization of polyolefin portion occurs next, releasing CO and CO2. Kishitani and Nakamura (1974) reported that the largest amount of HCN was evolved from urethane foam at 500°C (932°F), whereas with polyacrylonitrile and nylon 66, the amount of HCN increased with increasing temperature.
Uses
HCN was first isolated from a blue dye, Prussian blue, in 1704. HCN is obtainable from fruits that have a pit, such as cherries, apricots, and bitter almonds, from which almond oil and flavouring are made. HCN is used in fumigating, electroplating, mining, and producing synthetic fibres, plastics, dyes, and pesticides. It also is used as an intermediate in chemical syntheses.
Besides, hydrogen cyanide is used in manufacturing cyanide salts, aerylonitrile,and dyes.It is also used as a horticultural fumigant.
Uses
The high-tonnage uses of HCN are in the preparation of numerous chemical products and intermediates for organic syntheses. As a gas, HCN sometimes is applied as a disinfectant; or cellulosic disks impregnated with HCN may be used. In ore processing and metal treating, cyanides are widely used.
Production Methods
Hydrogen cyanide has been manufactured from sodium cyanide and mineral acid and from formamide by catalytic dehydration. Two synthesis processes account for most of the hydrogen cyanide produced. The dominant commercial process for direct production of hydrogen cyanide is based on classic technology involving the reaction of ammonia, methane (natural gas), and air over a platinum catalyst; it is called the Andrussow process. The second process, which involves the reaction of ammonia and methane, is called the Blaus€aure–Methan–Ammoniak (BMA) process; it was developed by Degussa in Germany. Hydrogen cyanide is also obtained as a by-product in the manufacture of acrylonitrile by the ammoxidation of propylene (Sohio process).
Definition
An addition compound formed between an aldehyde or ketone and hydrogen cyanide. The general formula is RCH(OH)(CN) (from an aldehyde) or RR′C(OH)(CN) (from a ketone). Cyanohydrins are easily hydrolyzed to hydroxycarboxylic acids. For instance, the compound 2-hydroxypropanonitrile (CH3CH(OH)(CN)) is hydrolyzed to 2-hydroxypropanoic acid (CH3CH(OH)(COOH)).
Production Methods
Hydrogen cyanide can be prepared from a mixture of NH3, methane, and air by partial combustion in the presence of a platinum catalyst: HN3 + CH4 + 1.5 O2 +6 N2 → HCN +3 H2O + 6N2 The process is carried out at about 900–1,000 °C; yield ranges from 55–60%. In another process, methane (contained in natural gas) is reacted with NH3 over a platinum catalyst at from 1,200–1,300 °C, the reaction requiring considerable heat input. In still another process, a mixture of methane and propane is reacted with NH3 : C3H8 + 3NH3 → 3HCN + 7H2; or CH4 + NH3 → HCN + 3H2. An electrically heated fluidized bed reactor is used. Reaction temperature is approximately 1,510 °C.
Definition
A highly poisonous weak acid formed when hydrogen cyanide gas dissolves in water. Its salts are cyanides. Hydrogen cyanide is used in making acrylic plastics.
Definition
prussic acid: A colourless liquidor gas, HCN, with a characteristicodour of almonds; r.d. 0.699 (liquid at22°C); m.p. –14°C; b.p. 26°C. It is anextremely poisonous substanceformed by the action of acids onmetal cyanides. Industrially, it is madeby catalytic oxidation of ammonia and methane with air and is used inproducing acrylate plastics. Hydrogencyanide is a weak acid (Ka = 2.1 × 10-9mol dm-3). With organic carbonylcompounds it forms cyanohydrins.
Preparation
Hydrogen cyanide is generally produced in industrial quantities by hightemperature catalytic reaction between ammonia, methane, and air (theAndrussow process). The stoichiometry of the process is:
2CH4 + 2NH3 + 3O2 → HCN + 3H2O ΔHrxn = 230.4 kcal
The above reaction is endothermic requiring a temperature of 1,100°C and acatalyst such as platinum or rhodium. Other hydrocarbons may be usedinstead of methane.
The compound may be made by several other methods, which include:1. Heating methanol and ammonia in the absence of air at elevated temperatures (600 to 950°C) using a catalyst:
CH3OH + NH3 → HCN + H2O + H2
2. Thermal decomposition of formamide at elevated temperatures and reduced pressure:
HCONH2 → HCN + H2O
3. Heating acetonitrile and ammonia at 1,100 to 1,300°C:
CH3CN + NH3 → 2HCN +2H2
4. Reaction of sodium cyanide or potassium cyanide or potassium ferrocyanide with a mineral acid:
NaCN + HCl → HCN + NaCl
K4Fe(CN)6 + 6HCl → 6HCN + 4KCl + FeCl2
Definition
ChEBI: A one-carbon compound consisting of a methine group triple bonded to a nitrogen atom. Also known as formonitrile, hydrogencyanide and prussic acid,HCN is a highly toxic liquid that has the odor of bitter almonds and boils at 25.6 °C.
also known as hydrocyanic acid, prussic acid, and fonnonitrile, is a very poisonous colorless gas with a characteristic fragrance of bitter almonds. Small amounts of hydrogen cyanide derivatives in combination with glucose and benzaldehyde are found in nature in apricot,peach,cherry, and plum pits.It liquifies at 26°C (79 OF) and is soluble in water,alcohol,and ether. Hydrogen cyanide is usually sold commercially as an aqueous solution containing 2 to 10% hydrogen cyanide. HCN reacts with amines, oxidisers, acids, sodium hydroxide, calcium hydroxide, sodium carbonate, caustic substances, and ammonia. The aqueous solutions of hydrogen cyani dedecompose slowly to form anunonium formate. In some uses, it is preferable to generate hydrogen cyanide as needed, thus eliminating handling and storage problems.
Reactions
Hydrogen cyanide reacts with hydrogen at 140 °C in the presence of a catalyst, e.g., platinum black, to form methyl amine CH3NH2. When burned in air, it produces a pale violet flame; when heated with dilute sulfuric acid, it forms formamide HCONH2 and ammonium formate HCOONH4; when exposed to sunlight with chlorine it forms cyanogen chloride CNCl, plus hydrogen chloride. An important reaction of hydrogen cyanide is that with aldehydes or ketones, whereby cyanhydrins are formed, e.g., acetaldehyde cyanhydrin CH3CHOH·CH, and the resulting cyanhydrins are readily converted into alpha-hydroxy acids, e.g., alphahydroxypropionic acid CH3·CHOH·COOH.
General Description
Hydrocyanic acid solution is water containing up to 5% dissolved hydrocyanic acid with the faint odor of almonds. HYDROGEN CYANIDE is toxic by inhalation and skin absorption. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Its vapors are just barely lighter than air.
Reactivity Profile
This particular record contains hydrogen cyanide dissolved in water. Hydrogen cyanide is a very volatile liquid or colorless gas smelling of bitter almonds, b.p. 26° C. A deadly human poison by all routes. The gas (hydrogen cyanide) forms explosive mixtures with air, HYDROGEN CYANIDE reacts violently with acetaldehyde. HYDROGEN CYANIDE is a severe explosion hazard when heated or exposed to oxidizers. HYDROGEN CYANIDE may polymerize explosively at elevated temperature (50-60° C) or in the presence of traces of alkali [Wohler, L. et al., Chem. Ztg., 1926, 50, p. 761, 781]. In the absence of a stabilizer (e.g., phosphoric acid) HYDROGEN CYANIDE may undergo explosively rapid spontaneous (autocatalytic) polymerization leading to a fire. The reaction is autocatalytic because of ammonia formation. The anhydrous acid should be stabilized by the addition of acid. [Bond, J., Loss Prev. Bull., 1991, 101, p.3]. During the preparation of imidoester hydrochlorides, hydrogen chloride was rapidly passed over alcoholic hydrogen cyanide. An explosion ensued, even with cooling of the process, [J. Org. Chem., 1955, 20, 1573].
Hazard
Flammable, dangerous fire risk, explosive limits in air 6–41%. Toxic by ingestion, inhalation, and skin absorption. TLV: ceiling 4.7 ppm.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Health Hazard
Exposures to hydrogen cyanide cause adverse health effects to animals and humans. Hydrogen cyanide is readily absorbed from the lungs and the symptoms of poisoning begin within seconds to minutes. The symptoms of toxicity and poisoning include, but are not restricted to, asphyxia, lassitude or weakness, exhaustion, headache, confusion, nausea, vomiting, increased rate and depth of respiration, or respiration slow and gasp- ing, thyroid and blood changes. Inhalation of hydrogen cyanide causes headache, dizzi- ness, confusion, nausea, shortness of breath, convulsions, vomiting, weakness, anxiety, irregular heart beat, tightness in the chest, and unconsciousness, and these effects may be delayed. The target organs of induced toxicity and poisoning include the CNS, cardiovas- cular system, thyroid, and blood.
Health Hazard
Hydrogen cyanide is a dangerous acute poison by all toxic routes. Acute inhalation may cause death in seconds. Lethal effects due to inhalation of its vapor depend on its concentration in air and time of exposure. Inhalation of 270 ppm HCN in air can be fatal to humans instantly, while 135 ppm can cause death after 30 minutes (Patty 1963; ACGIH 1986). Exposure to high concentration can cause asphyxia and injure the central nervous system, cardiovascular system, liver, and kidney.
HCN is extremely toxic via ingestion, skin absorption, and ocular routes. Swallowing 50 mg can be fatal to humans. The symptoms of HCN poisoning at lethal dosage are labored breathing, shortness of breath, paralysis, unconsciousness, convulsions, and respiratory failure. At lower concentrations toxic effects are headache, nausea, and vomiting.
LD50 value, intravenous (mice): 0.99 mg/kg
LD50 value, oral (mice): 3.70 mg/kg
Investigating the relationship between pH (in the range 6.8–9.3) and the acute toxicity of HCN on fathead minnow, Broderius et al. (1977) observed that similar to H2S, the toxicity of HCN increased at an elevated pH value. This was attributed to certain chemical changes occurring at the gill surface and possible penetration of the gill by both molecular and anionic forms.
In an acute lethal toxicity study on the influence of exposure route, Ballantyne (1983a) observed that the blood cyanide concentrations varied with the route. Concentrations in certain specific tissues varied markedly with exposure route. The blood cyanide concentration was lowest by inhalation and skin penetration. For a given exposure route, the cyanide level in blood were similar for different species. Among the most toxic cyanides, HCN was more toxic than NaCN or KCN by intramuscular and transocular routes.
Blank et al. (1983) carried out inhalation toxicity studies of hydrogen cyanide on Sprague-Dawley rats. Exposure at 68 ppm HCN in air 6 hours per day for three consecutive days showed symptoms of hypoactivity, breathing difficulties, signs of hypoxia, convulsions, and chromorhinorrhea. Death resulted in three of the five male rats after 1 day of exposure, caused by cyanosis of the extremities, moderate to severe hemorrhage of the lung, and pulmonary edema. All female rats survived. In a 4-week study, no mortality was observed at concentrations up to 58 ppm HCN. A brief exposure to 125 ppm HCN for 15 minutes, however, was fatal to 20% of the test animals. Increased urine thiocyanate levels were observed in test animals However, no adverse effects were observed in rats exposed at 29 ppm HCN 6 hour’s weekday in 4-week studies.
Alarie and Esposito (1988) proposed a blood cyanide concentration of 1 mg/L as the fatal threshold value for HCN poisoning by inhalation. A cyanide concentration of 1.2 mg/L was measured in test animals exposed to nylon carpet smoke. The combined toxicities of fire-effluent gases CO and HCN was found to be additive (Levin et al. 1988). The study indicated that the sublethal concentrations of the individual gases became lethal when combined. Furthermore, the presence of CO2 combined with decreasing oxygen concentration enhanced the toxicity of the CO–HCN mixture (Levin et al. 1987). HCN and nitric oxide hastened the incapacitation in rats produced by carbon monoxide. Such incapacitation occurred at a carbonyl hemoglobin concentration of 42.2–49%; while for CO alone 50–55% carbonyl hemoglobin manifested the same effect (Conditet al. 1978).
Health Hazard
HCN is particularly dangerous because of its toxic and asphyxiating effects on all life requiring oxygen to survive. HCN combines with the enzymes in tissue associated with cellular oxidation. The signs and symptoms of HCN poisoning are non-specifi c and very rapid. The symptoms include excitement, dizziness, nausea, vomiting, headache, weakness, drowsiness, gasping, thyroid, blood changes, confusion, fainting, tetanic spasm, lockjaw, convulsions, hallucinations, loss of consciousness, coma, and death. When oxygen becomes unavailable to the tissues, it leads to asphyxia and causes death. Children are more vulnerable to HCN exposure. HCN is readily absorbed from the lungs; symptoms of poisoning begin within seconds to minutes. Inhalation of HCN results in the rapid onset of poisoning, producing almost immediate collapse, respiratory arrest, and death within minutes (Table 1)
Health Hazard
The acute toxicity of hydrogen cyanide is high, and exposure by inhalation, ingestion, or eye or skin contact can be rapidly fatal. Symptoms observed at low levels of exposure (e.g., inhalation of 18 to 36 ppm for several hours) include weakness, headache, confusion, nausea, and vomiting. Inhalation of 270 ppm can cause immediate death, and 100 to 200 ppm can be fatal in 30 to 60 min. Aqueous solutions of HCN are readily absorbed through the skin and eyes, and absorption of 50 mg can be fatal. In humans, ingestion of 50 to 100 mg of HCN can be fatal. Because there is wide variation in the ability of different individuals to detect the odor of HCN, this substance is regarded as having poor warning properties. Effects of chronic exposure to hydrogen cyanide are nonspecific and rare
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Fire Hazard
Hydrogen cyanide is a highly flammable liquid. Liquid HCN contains a stabilizer (usually phosphoric acid), and old samples may explode if the acid stabilizer is not maintained at a sufficient concentration.
Flammability and Explosibility
Hydrogen cyanide is a highly flammable liquid. Liquid HCN contains a stabilizer (usually phosphoric acid), and old samples may explode if the acid stabilizer is not maintained at a sufficient concentration.
Potential Exposure
AgriculturalChemical; Human Data. Hydrogen cyanide is used in chem-ical synthesis of sequestrants, polymers, weed killers, andpharmaceuticals; as a fumagant; in electroplating, mining,chemical synthesis, and the production of synthetic fibers,plastics, dyes, and pesticides; in chemical synthesis of acry-lates and nitriles, particularly acrylonitrile. It may be gener-ated in blast furnaces, gas works, and coke ovens. Cyanidesalts have a wide variety of uses, including steel hardening,gold and silver extraction from ores. AC is used as a chemi-cal warfare agent (blood agent); systemic agent. It formscyanide in the body.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Useamyl nitratecapsules if symptoms develop. Allareaemployees should be trained regularly in emergency mea-sures for cyanide poisoning and in CPR. A cyanide antidotekit should be kept in the immediate work area and must berapidly available. Kit ingredients should be replaced every1-2 years to ensure freshness. Persons trained in the use ofthis kit, oxygen use, and CPR must be quickly available.
storage
Hydrogen cyanide should be stored in a cool, dry, well-ventilated area in tightly sealed containers and with the correct label. Containers of hydrogen cyanide should be protected from physical damage and should be stored separately from amines and oxidizers, such as perchlorates, peroxides, permanganates, chlorates, and nitrates. It should be kept sepa- rated from strong acids, such as hydrochloric, sulfuric, and nitric acids, away from sodium hydroxide, calcium hydroxide, sodium carbonate, water, ammonia, acetaldehyde, and caustics.
Shipping
Hydrocyanicacid,aqueoussolutionsorHydrogen cyanide, solutions with not > 20% hydrogen cya-nide requires a shipping label of“POISONOUS/TOXIC .MATERIALS." It falls in Hazard Class 6.1 and PackingGroup I.Hydrogen cyanide, stabilized, with <3% water requires ashipping label of“POISONOUS/TOXIC MATERIALS,FLAMMABLE LIQUID.”" It falls in Hazard Class 6.1 andPacking Group I.Hydrogen cyanide,stabilized, with <3%water andabsorbed in a porous inert material requires a shipping labelof“POISONOUS/TOXIC MATERIALS." It falls in HazardClass 6.1 and Packing Group I.
Purification Methods
HCN is prepared from NaCN and H2SO4, and dried by passage through H2SO4 and over CaCl2, then distilled in a vacuum system and degassed at 77oK before use [Arnold & Smith J Chem Soc, Faraday Trans 2 77 861 1981]. Cylinder HCN may contain stabilisers against explosive polymerisation, together with small amounts of H3PO4, H2SO4, SO2, and water. It can be purified by distillaton over P2O5, then frozen in Pyrex bottles at Dry-ice temperature for storage. [Zeigler Org Synth Coll Vol I 314 1941, Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 658-660 1963.] Liquid HCN, like liquid ammonia, evaporates very slowly since the latent heat of evaporation is high and keeps it in the liquid state because the temperature of the liquid is lowered to below its boiling point. EXTREMELY POISONOUS; all due precautions should be taken.
Incompatibilities
HCN can polymerize explosively if heated above 50 °C or in the presence of trace amounts of alkali.
Waste Disposal
In the event of a spill, remove all ignition sources. Cleanup should be conducted wearing appropriate chemical-resistant clothing and respiratory protection Disposal Excess hydrogen cyanide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. For more information on disposal procedures, see Chapter 7 of this volume.
Precautions
Occupational workers should be very careful in the management of HCN since the gas in air is explosive at concentrations over 5.6%, equivalent to 56,000 ppm and it does not provide adequate warning of hazardous concentrations. HCN at a concentration of 300 mg/m3 in air becomes fatal within about 10 min and HCN at a concentration of 3500 ppm (about 3200 mg/m3 ) kills a human in about 1 min.
HYDROGEN CYANIDE Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
Related articles
- Drawing of the HCN Lewis structure
- The HCN Lewis structure consists of three atoms: hydrogen, carbon and nitrogen.
- Nov 9,2023
74-90-8(HYDROGEN CYANIDE)Related Search:
1of4