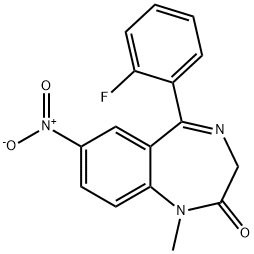
FLUNITRAZEPAM
- CAS No.
- 1622-62-4
- Chemical Name:
- FLUNITRAZEPAM
- Synonyms
- Rohypnol;Primum;Silece;Roipnol;Narcozep;FlunipaM;RO 5-4200;Flnitrazepam;FlunidazepaM;FLUNITRAZEPAM
- CBNumber:
- CB5111857
- Molecular Formula:
- C16H12FN3O3
- Molecular Weight:
- 313.28
- MDL Number:
- MFCD00057906
- MOL File:
- 1622-62-4.mol
| Melting point | 166-167°; mp 170-172° |
|---|---|
| Boiling point | 540.9±50.0 °C(Predicted) |
| Density | 1.3720 (estimate) |
| Flash point | 11 °C |
| storage temp. | 2-8°C |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1 mg/mL |
| form | A neat solid |
| pka | pKa 1.8 (Uncertain) |
| EWG's Food Scores | 1 |
| FDA UNII | 620X0222FQ |
| ATC code | N05CD03 |
| EPA Substance Registry System | 2H-1,4-Benzodiazepin-2-one, 5-(2-fluorophenyl)-1,3-dihydro-1-methyl-7-nitro- (1622-62-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H319 | |||||||||
| Precautionary statements | P305+P351+P338 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 22-36-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 7-16-36/37-45-36-26 | |||||||||
| RIDADR | UN 1230 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | DF2385000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| NFPA 704 |
|
FLUNITRAZEPAM Chemical Properties,Uses,Production
Chemical Properties
White or yellowish, crystalline powder.
Originator
Roipnol,Roche,Italy,1976
Uses
antitussive
Uses
Flunitrazepam is used as an hypnotic. This is a controlled substance (depressant).
Definition
ChEBI: Flunitrazepam is a 1,4-benzodiazepinone that is nitrazepam substituted by a methyl group at position 1 and by a fluoro group at position 2'. It is a potent hypnotic, sedative, and amnestic drug used to treat chronic insomnia. It has a role as a sedative, a GABAA receptor agonist and an anxiolytic drug. It is a 1,4-benzodiazepinone, a C-nitro compound and a member of monofluorobenzenes.
Manufacturing Process
A mixture of 176 grams of orthofluorobenzoyl chloride and 64 grams of parachloroaniline
was stirred and heated to 180°C, at which temperature 87 grams
of zinc chloride was introduced, the temperature raised to 200° to 205°C and
maintained there for 40 minutes. The golden colored melt was quenched by
the careful addition of 500 ml of 3 N hydrochloric acid and the resulting
mixture refluxed for 5 minutes. The acid solution was decanted and the
process repeated three times to remove all orthofluorobenzoic acid. The grey
granular residue was dissolved in 300 ml of 75% (v/v) sulfuric acid and
refluxed for 40 minutes to complete hydrolysis. The hot solution was poured
over 1 kg of ice and diluted to 2 liters with water. The organic material was
extracted with four 300 ml portions of methylene chloride, and the combined
extracts subsequently washed with two 500 ml portions of 3 N hydrochloric
acid to remove traces of para-chloroaniline, three 500 ml portions of 5 N
sodium hydroxide solution to remove orthofluorobenzoic acid, and finally two
200 ml portions of saturated brine solution.
The combined methylene chloride extracts were dried over anhydrous sodium
sulfate and the solvent removed to give the crude 2-amino-5-chloro-2'-
fluorobenzophenone which upon recrystallization from methanol formed yellow
needles melting at 94° to 95°C.
50.0 grams of 2-amino-5-chloro-2'-fluorobenzophenone in 300 cc of
tetrahydrofuran was hydrogenated at atmospheric pressure in the presence of
10 grams of charcoal (Norite), 30.0 grams of potassium acetate and 2.5 cc of
a 20% palladous chloride solution (20% by weight of palladium). After an
initiation period varying from 10 minutes to an hour, hydrogen uptake was
rapid and stopped completely after the absorption of the theoretical amount.
Filtration of the catalyst over a Hyflo pad and removal of the solvent left a
yellow crystal line residue. The crude mixture of ketone and potassium
acetate was partitioned between methylene chloride (300 cc) and water (1
liter). The layers were separated and the water layer washed with methylene
chloride (3 x 50 cc). The organic layers were combined, washed with 3 N sodium hydroxide solution (2 x 50 cc), water (3 x 100 cc), dried over
anhydrous sodium sulfate and filtered. The solvent was removed and the
product recrystallized from ethanol to give 2-amino-2'-fluorobenzophenone as
yellow prisms melting at 126° to 128°C.
A solution of 21.5 grams of 2-amino-2'-fluorobenzophenone in 500 cc of ether
was treated with 20 cc of a 20% (v/v) solution of bromoacetyl bromide in
ether. The mixture was shaken and allowed to stand for 5 minutes and then
washed with water (20 cc). The process was repeated five times. The final
solution was washed thoroughly with water (5 x 500 cc) and concentrated to
100 cc. The crystals were filtered and recrystallized from methanol to give 2-
bromacetamido-2'-fluorobenzophenone as white needles melting at 117° to
118.5°C.
A solution of 23.7 grams of 2-bromoacetamido-2'-fluorobenzophenone in
tetrahydrofuran (100 cc) was added to liquid ammonia (approximately 500 cc)
and allowed to evaporate overnight. The residue was treated with water (1
liter) and the crystals filtered off and refluxed in toluene (100 cc) for 30
minutes. The mixture was treated with decolorizing carbon (Norite) and
filtered over Hyflo. The solution was concentrated to a small volume (25 cc)
cooled, diluted with 20 cc of ether and allowed to stand. The product was
recrystallized from acetone/hexane to give 5-(2-fluorophenyl)-3H-1,4-
benzodiazepin-2(1H)-one as white needles melting at 180° to 181°C.
23.8 grams of 5-(2-fluorophenyl)-3H-1,4-benzodiazepin-2(1H)-one was
dissolved in 50 cc of concentrated sulfuric acid at 0°C. To the resulting
mixture there was then added dropwise with stirring a solution of 7.1 grams
of potassium nitrate in 20 cc of concentrated sulfuric acid. The mixture was
stirred for 2? hours at 0°C and then diluted with 300 grams of ice. The
resulting solution was made alkaline with concentrated ammonium hydroxide
solution, keeping the temperature at 0°C. The formed suspension was
extracted thoroughly with methylene chloride (6 x 100 cc). The organic layers
were combined, washed with saturated brine solution, dried over anhydrous
sodium sulfate and filtered. Removal of the solvent yielded a brown gum
which was taken up in a small amount of methylene chloride and filtered
through a pad of grade I alumina. The alumina was eluted with methylene
chloride, the solvent removed, and the residue crystallized from
acetone/hexane to yield 7-nitro-5-(2-fluorophenyl)-3H-1,4-benzodiazepin-
2(1H)-oneas white needles melting at 210° to 211°C.
23.8 grams of 5-(2-fluorophenyl)-3H-1,4-benzodiazepin-2(1H)-one was
dissolved in 50 cc of concentrated sulfuric acid at 0°C. To the resulting
20.2 grams of the abovementioned 7-nitro-5-(2-fluorophenyl)-3H-1,4-
benzodiazepin-2(1H)-one was dissolved in 60 cc of N,N-dimethyl formamide to
which was then added 3.49 grams of a 50% suspension of sodium hydride in
heavy mineral oil. The mixture was allowed to stir for 15 minutes in the cold,
11.2 grams of methyl iodide was added and the solution was stirred for a
further 20 minutes. Solvent was removed under reduced pressure to give an
oil which was partitioned between water and methylene chloride (1 liter/300
cc), the water layer was extracted with methylene chloride (5 x 200 cc), the
organic layers combined and washed with water (2 x 100 cc), 3N hydrochloric
acid (1
brand name
Rohypnol (Roche, Puerto Rico);Darkene;Flumipam;Hipnosedon;Hiposedon;Hypnodorm;Hypnosedon;Libelins;Noviel;Primun;Riopnol;Rohipnol;Rohpinol;Rohpnol;Valseram.
Therapeutic Function
Hypnotic
World Health Organization (WHO)
Flunitrazepam, a benzodiazepine derivative with sedative and hypnotic activity, was introduced in 1974 for the management of anxiety. Although it is subject to international control under Schedule IV of the 1971 Convention on Psychotropic Substances, its potential for abuse by drug addicts has led at least two countries (Germany and Turkey) to apply controls equivalent to those of Schedule II. (Reference: (UNCPS4) United Nations Convention on Psychotropic Substances (IV), , , 1971)
Biochem/physiol Actions
Hypnotic; anxiolytic; ligand for the GABAA receptor benzodiazepine modulatory site.
FLUNITRAZEPAM Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1622-62-4(FLUNITRAZEPAM)Related Search:
1of3