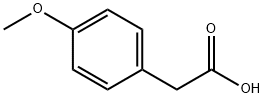
4-Methoxyphenylacetic acid
- CAS No.
- 104-01-8
- Chemical Name:
- 4-Methoxyphenylacetic acid
- Synonyms
- P-METHOXYPHENYLACETIC ACID;4MPAA;HOMOANISIC ACID;ASISCHEM D13364;HOMO-P-ANISIC ACID;RARECHEM AL BO 0133;p-Anisylacetic acid;4-Methoxyphenylaceti;p-Methioxy-toluicacid;4-Methoxyphenylacetic
- CBNumber:
- CB5113770
- Molecular Formula:
- C9H10O3
- Molecular Weight:
- 166.17
- MDL Number:
- MFCD00004345
- MOL File:
- 104-01-8.mol
- MSDS File:
- SDS
| Melting point | 84-86 °C(lit.) |
|---|---|
| Boiling point | 140 °C3 mm Hg(lit.) |
| Density | 1.1708 (rough estimate) |
| vapor pressure | 0.071Pa at 25℃ |
| refractive index | 1.5101 (estimate) |
| Flash point | 193°C |
| storage temp. | Store below +30°C. |
| solubility | 6g/l |
| pka | pK1:4.358 (25°C) |
| form | Crystalline Powder and Chunks |
| color | White to light yellow |
| Water Solubility | 6 g/L (20 ºC) |
| BRN | 1101737 |
| InChIKey | NRPFNQUDKRYCNX-UHFFFAOYSA-N |
| LogP | 1.295 at 25℃ |
| CAS DataBase Reference | 104-01-8(CAS DataBase Reference) |
| FDA UNII | AJP2V8U5K6 |
| NIST Chemistry Reference | Benzeneacetic acid, 4-methoxy-(104-01-8) |
| EPA Substance Registry System | Benzeneacetic acid, 4-methoxy- (104-01-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H318 | |||||||||
| Precautionary statements | P280-P305+P351+P338 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | AI8960000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29189090 | |||||||||
| NFPA 704 |
|
4-Methoxyphenylacetic acid price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | M19201 | 4-Methoxyphenylacetic acid ReagentPlus , 99% | 104-01-8 | 5g | $45.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | M19201 | 4-Methoxyphenylacetic acid ReagentPlus , 99% | 104-01-8 | 100g | $71.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.21240 | 4-Methoxyphenylacetic acid for synthesis | 104-01-8 | 100g | $76.5 | 2022-05-15 | Buy |
| TCI Chemical | M0742 | 4-Methoxyphenylacetic Acid >98.0%(GC)(T) | 104-01-8 | 25g | $20 | 2024-03-01 | Buy |
| TCI Chemical | M0742 | 4-Methoxyphenylacetic Acid >98.0%(GC)(T) | 104-01-8 | 100g | $34 | 2024-03-01 | Buy |
4-Methoxyphenylacetic acid Chemical Properties,Uses,Production
Uses
4-Methoxyphenylacetic Acid is a compound that was found from microbial phenolic metabolites in human feces after the consumption of gin, red wine and dealcholized red wine.
Description
4-METHOXYPHENYLACETIC ACID(104-01-8) is used in organic synthesis as well as pharmaceutical industry, especially used as an intermediate of puerarin. It can also be used as potential plasma biomarkers for early detection of non-small cell lung cancer. It is also used in drug industry as an intermediate to make Dextromethorphan.
Reference
Chen, Yan Ping, Y. M. Chen, and M. Tang. "Solubilities of cinnamic acid, phenoxyacetic acid and 4-methoxyphenylacetic acid in supercritical carbon dioxide." Fluid Phase Equilibria 275.1(2008):33-38.
Chemical Properties
white to light yellow crystalline powder and
Uses
4-Methoxyphenylacetic acid can be used:
- To prepare methyl 4-methoxyphenylacetate by esterification with dimethyl carbonate using mesoporous sulfated zirconia catalyst.
- As a ligand to synthesize pharmacologically important dinuclear gallium(III) and phenyltin(IV) carboxylate metal complexes.
- As a reactant to synthesize hydroxylated (E)-stilbenes by reacting with substituted benzaldehydes via Perkin reaction.
Uses
4-Methoxyphenylacetic Acid is a compound that was found from microbial phenolic metabolites in human feces after the consumption of gin, red wine and dealcholized red wine.
Uses
Preparation of pharmaceuticals, other organic compounds.
Definition
ChEBI: A monocarboxylic acid that is phenylacetic acid carrying a 4-methoxy substituent. It is used as an intermediate for pharmaceuticals and other organic synthesis. It has been found to inhibit the germination of cress and lettuce seeds.
Synthesis Reference(s)
The Journal of Organic Chemistry, 51, p. 4354, 1986 DOI: 10.1021/jo00373a005
Tetrahedron Letters, 35, p. 133, 1994 DOI: 10.1016/0040-4039(94)88182-0
General Description
Pale yellow or off white colored flakes. Severely irritates skin and eyes. May be toxic by ingestion.
Reactivity Profile
Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 4-Methoxyphenylacetic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Not classified
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. Questionable carcinogen with experimental neoplastigenic data. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Crystallise the acid from EtOH/water, EtOAc/pet ether (m 87o) or *C6H6/pet ether (m 84-86o). [Beilstein 10 III 431, 10 IV 544.] The acid chloride [4693-91-8] has M 184.6, b 143o/10mm, d 254
4-Methoxyphenylacetic acid Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | sales@finerchem.com | China | 2967 | 58 |
| Accela ChemBio Inc. | (+1)-858-699-3322 | info@accelachem.com | United States | 19965 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8823 | 58 |
View Lastest Price from 4-Methoxyphenylacetic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-03-27 | 4-Methoxyphenylacetic acid
104-01-8
|
US $0.00-0.00 / kg | 1kg | 99% | 50000kg | Hebei Kingfiner Technology Development Co. , Ltd. | |
 |
2023-09-12 | 4-Methoxyphenylacetic acid
104-01-8
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-19 | 4-Methoxyphenylacetic acid
104-01-8
|
US $30.00 / KG | 1KG | 99% | 20 Tons | Wuhan wingroup Pharmaceutical Co., Ltd |
-

- 4-Methoxyphenylacetic acid
104-01-8
- US $0.00-0.00 / kg
- 99%
- Hebei Kingfiner Technology Development Co. , Ltd.
-

- 4-Methoxyphenylacetic acid
104-01-8
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- 4-Methoxyphenylacetic acid
104-01-8
- US $30.00 / KG
- 99%
- Wuhan wingroup Pharmaceutical Co., Ltd