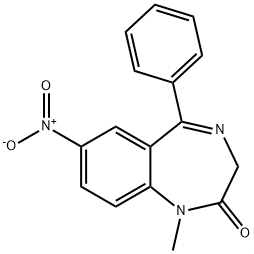
Nimetazepam
- CAS No.
- 2011-67-8
- Chemical Name:
- Nimetazepam
- Synonyms
- Elimin;Hypnon;S 1530;ro5-3453;Nimetazam;NIMETAZEPAM;N-Methylmogadon;Nimetazepam (CRM);1-Methylnitrazepam;Nimetazepam solution
- CBNumber:
- CB6139366
- Molecular Formula:
- C16H13N3O3
- Molecular Weight:
- 295.29
- MDL Number:
- MFCD00242916
- MOL File:
- 2011-67-8.mol
| Melting point | 156.5-157.5° |
|---|---|
| Boiling point | 436.98°C (rough estimate) |
| Density | 1.1952 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| Flash point | 9℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 2.53(EtOH/H2O,t =25,Iundefined) (Uncertain) |
| color | Light Yellow to Dark Yellow |
| CAS DataBase Reference | 2011-67-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 4532264KW6 |
| ATC code | N05CD15 |
| NIST Chemistry Reference | Nimetazepam(2011-67-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H301+H311+H331-H370 | |||||||||
| Precautionary statements | P210-P260-P280-P301+P310-P311 | |||||||||
| Hazard Codes | F,T | |||||||||
| Risk Statements | 11-23/24/25-39/23/24/25 | |||||||||
| Safety Statements | 16-36/37-45 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 1 | |||||||||
| Toxicity | LD50 in male, female mice, rats (mg/kg): 910, 750, 1150, 970 orally; 970, 840, 970, 980 i.p.; 1500, 1500, 1000, 1000 s.c. (Sakai) | |||||||||
| DEA Controlled Substances | CSCN: 2837 CSA SCH: Schedule IV NARC: No |
|||||||||
| NFPA 704 |
|
Nimetazepam Chemical Properties,Uses,Production
Chemical Properties
Dark yellow Solid
Originator
Erimin,Sumitomo,Japan,1977
Uses
Sedative, hypnotic. Controlled substance.
Definition
ChEBI: A nitrazepam which is substituted at positions 1 by a methyl groups. It is used as an anticonvulsant and as a hypnotic for the short-term management of insomnia.
Manufacturing Process
To a suspension of 73.9 g of 1-methyl-5-nitro-3-phenylindole-2-carbonitrile in
1.5 liters of dry tetrahydrofuran is added dropwise a solution of 126 g of
boron trifluoride etherate in 220 ml of dry tetrahydrofuran with stirring for 2
hours. After addition, stirring is continued for an additional 3 hours. To the reaction mixture is added dropwise 370 ml of water and then 370 ml of
concentrated hydrochloric acid with stirring under ice-cooling.
The resulting precipitate is collected by filtration, washed with water followed
by ethanol, and dried to give 56.3 g of crude 2-aminomethyl-1-methyl-5-
nitro-3-phenylindole hydrochloride, melting point 263°C to 267°C.
To a suspension of 6.5 g of 2-aminomethyl-1-methyl-5-nitro-3-phenylindole in
65 ml of glacial acetic acid is added dropwise a solution of 6.5 g of chromic
anhydride in 6.5 ml of water at 20°C with stirring. The mixture is stirred at
room temperature overnight and thereto is added 195 ml of water. To the
mixture is added dropwise 100 ml of 28% ammonia water with stirring under
cooling. The resultant precipitate is collected by filtration, washed with water
and dried to give 5.9 g of a crude product having melting point 135°C to
140°C. Fractional recrystallization from ethanol gives 3.8 g of 1-methyl-7-
nitro-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepine-2-one as yellow plates,
melting point 153°C to 156°C. Further recrystallization from the same solvent
gives pale yellow plates having melting point 156°C to 156.5°C.
Therapeutic Function
Tranquilizer