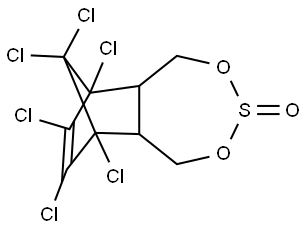
Thiosulfan
- CAS No.
- 115-29-7
- Chemical Name:
- Thiosulfan
- Synonyms
- ENDOSULFAN;thionate;Benzoepin;ENDOSULFANE;FAN;3-oxide;6,7,8,9,10,10-Hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzo[e]-dioxathiepin-3-oxide;Hilda;oms570;ensure
- CBNumber:
- CB6153380
- Molecular Formula:
- C9H6Cl6O3S
- Molecular Weight:
- 406.93
- MDL Number:
- MFCD00137651
- MOL File:
- 115-29-7.mol
- MSDS File:
- SDS
| Melting point | 106°C |
|---|---|
| Boiling point | 106 °C(Press: 0.70 Torr) |
| Density | 1.7450 |
| vapor pressure | 8.3 x 10-4 Pa (25 °C) for 2:l mixture of α- and β-isomers |
| Flash point | -26 °C |
| storage temp. | APPROX 4°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Sparingly) |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 13,3608 |
| BRN | 2062338 |
| CAS DataBase Reference | 115-29-7(CAS DataBase Reference) |
| EWG's Food Scores | 4-5 |
| NCI Dictionary of Cancer Terms | Ensure |
| FDA UNII | OKA6A6ZD4K |
| NIST Chemistry Reference | Endosulfan(115-29-7) |
| NCI Drug Dictionary | Ensure |
| EPA Substance Registry System | Endosulfan (115-29-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310+H330-H410 |
| Precautionary statements | P260-P262-P273-P280-P302+P352+P310-P304+P340+P310 |
| Hazard Codes | T,N,Xn,F,T+ |
| Risk Statements | 24/25-36-50/53-67-65-62-51/53-48/20-38-11-26/28-21 |
| Safety Statements | 28-36/37-45-60-61-62-63-33-29-16-9 |
| RIDADR | UN 2761 |
| WGK Germany | 3 |
| RTECS | RB9275000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| HS Code | 29209090 |
| Toxicity | LD50 orally in male, female rats: 43, 18 mg/kg (Gaines) |
Thiosulfan price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 32015 | Endosulfan PESTANAL?, analytical standard | 115-29-7 | 250mg | $72.6 | 2024-03-01 | Buy |
| TRC | E555505 | Endosulfan(MixtureofDiastereomers) | 115-29-7 | 10g | $275 | 2021-12-16 | Buy |
| Usbiological | 448215 | Endosulfan | 115-29-7 | 100mg | $319 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PST0000159 | ENDOSULFAN 95.00% | 115-29-7 | 250MG | $597.64 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 40265 | Endosulfan(MixtureofDiastereomers) | 115-29-7 | 1g | $610 | 2021-12-16 | Buy |
Thiosulfan Chemical Properties,Uses,Production
Description
Endosulfan (70) [115-29-7], 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3,- benzo-dioxathiepine 3-oxide (IUPAC) [The technical product is a mixture of two isomers: α-endosulfan: 3α,5αβ,6α, 9α,9αβ (64–67%) (70a) and β-endosulfan 3α,5aα,6β,9β, 9aα, (29–32%) (70b)]; [959-98-8] (formerly [33213-66- 0]) (β-endosulfan);[33213-65-9] (formerly [891-86-1] and [19670-15-6]) (β-endosulfan) is the adduct of hexachlorocyclopentadiene and 1,4-dihydroxy-2-butene reacted further with SOCl2 to produce 6,7,8,9,10,10-hexachloro- 1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxa thiepin-3-oxide. The technical product is a brownish solid, mp 70–100 ?C, vapor pressure 1.3 mPa at 25 ?C, soluble in petroleum solvents but having low solubility in water. It consists of about four parts of α-isomer (mp 108 ?C, cis with regard to the sulfite group) and one part of the β-isomer (mp 206 ?C, trans with regard to the sulfite group). The α-isomer, which is somewhat more insecticidal, is slowly converted to the more stable β-isomer at high temperature, and both isomers are oxidized slowly to endosulfan sulfate [1031-07-8] (mp 181 ?C). In acid media, both isomers form endosulfan diol [2157-19-9] (mp 203 ?C).
Chemical Properties
Endosulfan is a chlorinated cyclodiene insecticide. The pure product is a colorless crystalline solid. The technical product is a light to dark brown waxy solid. It has a rotten egg or sulfur odor.
Chemical Properties
Endosulfan is a pesticide. It is a creamto brown-colored solid that may appear in the form of crystals or fl akes. It has a smell like turpentine, but does not burn. It does not occur naturally in the environment. Endosulfan is used to control insects on food and non-food crops and also as a wood preservative. Endosulfan is used for the control of ticks and mites, and the control of rice stem borers. It is an RUP, meaning it can only be used by professional applicators. Endosulfan, commonly known by its trade name Thiodan, is an insecticide and was fi rst introduced in the 1950s. Endosulfan enters the air, water, and soil during its manufacture and use. It is often sprayed onto crops and the spray may travel long distances before it lands on crops, soil, or water. On crops, endosulfan usually breaks down in a few weeks, but it sticks to soil particles and may take years to completely break down. Endosulfan does not dissolve easily in water. In surface water, endosulfan attaches to soil particles fl oating in water or attaches to soil at the bottom. It can build up in the bodies of animals that live in endosulfan- contaminated water. It is also extremely toxic to fi sh and other aquatic life. Exposures to endosulfan occur among workers and people working in industries involved in making endosulfan or as pesticide applicators and by skin contact with soil containing endosulfan.
Chemical Properties
(commercial product): Brown crystals. Mixture of two isomers.
Uses
Endosulfan is used for the control of sucking, chewing, boring insects and mites on a wide variety of crops. It also controls tsetse flies.
Uses
Insecticide.
Definition
ChEBI: A cyclic sulfite ester that is 1,5,5a,6,9,9a-hexahydro-6,9-methano-2,4,3-benzodioxathiepine 3-oxide substituted by chloro groups at positions 6, 7, 8, 9, 10 and 10.
General Description
Endosulfan is a pesticide. It is a cream- to brown-coloured solid that may appear in the form of crystals or flakes. It has a smell like turpentine, but does not burn. It does not occur naturally in the environment. It is a restricted use pesticide, meaning that it can only be used by professional applicators.
Air & Water Reactions
Sightly soluble in water. Slowly hydrolyzes to form sulfur dioxide and a diol; hydrolyzes more rapidly under basic or acidic conditions.
Reactivity Profile
Thiosulfan is an organochlorine, cyclodiene derivative. Thiosulfan is also a sulfite ester. Halogenated aliphatic or cyclic alkane compounds are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. As Thiosulfan is rather highly substituted Thiosulfan may be resistant to reaction. However, materials in this group are incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. As an ester, Thiosulfan will hydrolyze to form sulfur dioxide and diol; reaction is more rapid under basic conditions.
Hazard
Toxic by ingestion, inhalation, and skin absorption; use may be restricted. Lower respiratory tract irritant; liver and kidney damage. Questionable carcinogen.
Health Hazard
Endosulfan is readily absorbed by the stomach, the lungs, and through the skin, meaning that all routes of exposure can pose a hazard. Exposures to endosulfan cause adverse health effects and poisoning. The symptoms of toxicity include, but are not limited to, hyperactivity, nausea, dizziness, headache, irritability, restlessness, muscular twitching, and convulsions have been observed in adults exposed to high doses. Severe exposures cause poisoning, disturbances of the CNS, and may result in death. Laboratory studies in experimental animals indicated that long-term exposure to endosulfan can also damage the kidneys, testes, and liver and may possibly affect the body’s ability to fi ght infection. More studies are needed to confi rm similar situations in humans. Reports have indicated that the most prominent signs of acute exposure are hyperactivity, tremors, decreased respiration, diffi culty in breathing, salivation, and convulsions. Long-term neurotoxic effects have been observed after high acute exposure. The National Poison Control Information Center of the Philippines recorded 278 poisonings, including 85 deaths due to endosulfan in 1990. In Colombia, in 1993, at least 60 people were poisoned and one person died as a result of exposure to thiodan.
Health Hazard
Highly toxic; exhibits acute, delayed,and chronic toxicity; symptoms includenausea, vomiting, weakness, somnolence,excitement, and muscle contraction; causesdermatitis and irritation on skin contact; astimulant to central nervous system, producing convulsions; can cause adverse reproductive effects; no evidence of carcinogenicityin rats and mice; oral LD50 value (mice):~7.5 mg/kg; exposure limit: PEL/TLV-TWA(skin) 0.1 mg/m3 (OSHA:ACGIH); RCRAWaste Number P050; US EPA listedextremely hazardous substance.
Oktay et al. (2003) have reported a case ofendosulfan poisoning associated with neurological symptoms in two patients after eatingcontaminated food in Turkey.
Health Hazard
Thiosulfan is very toxic. The probable oral lethal dose is 50 to 500 mg/kg, or 1 teaspoonful to 1 ounce for a 150 lb. person.
Fire Hazard
Container may explode in heat of fire. Fire or run off from fire control water may release irritating or poisonous gases. Slowly oxidizes in air. Do not store at temperature below 20F.
Agricultural Uses
Insecticide, Acaricide: A U.S. EPA restricted Use Pesticide (RUP). Not approved for use in EU countries. Globally banned as of April 29, 2010. Endosulfan was added to the list of Stockholm Convention Persistent Organic Pollutants (POPs): Annex A (Elimination). Endosulfan is a chlorinated hydrocarbon insecticide and acaricide of the cyclodiene subgroup which acts as a poison to a wide variety of insects and mites on contact. Although it may also be used as a wood preservative, it is used primarily on a wide variety of food crops including tea, coffee, fruits, and vegetables, as well as on rice, cereals, maize, sorghum, or other grains. Formulations of endosulfan include emsulsifiable concentrate, wettable powder, ultra-low volume (ULV) liquid, and smoke tablets. It is compatible with many other pesticides and may be found in formulations with dimethoate, malathion, methomyl, monocrotophos, pirimicarb, triazophos, fenoprop, parathion, petroleum oils, and oxine-copper. It is not compatible with alkaline materials. Technical endosulfan is made up of a mixture of two molecular forms (isomers) of endosulfan, the alpha-and beta-isomers.
Trade name
AFIDEN®; BEOSIT®; BIO 5,462®; CHLORTHIEPIN®; CLEAN-CROP®; CRISUFAN®; CYCLODAN®; DE-PESTER®; DESTROY®; DEVISULPHAN®; DISSULFAN CE®; ENDOCEL® ENDOCIDE®; ENDOSOL®; END-O-SULFAN®; ENDOTAF®; ENDOX®; ENSURE®; E-Z FLO®; FMC 5462®; HEXASULFAN®; HILDAN®; HOE 2671®; INSECTO®; INSECTOPHENE®; KENDAN®; KERNTOX®; KOP-THIODAN®; MALIX®; MALUX; MAUX®; MOS-570; METHOFAN®; NCI-C00566; NIA 5462®, NIAGARA 5,462; NIAGARA 5,462®[C]; PHASER®; RASAYANSULFAN; ROCKY®; THIFOR®; THIDAN®; THIMUL®; THIODAN®; α-THIODAN®; β-THIODAN®; THIONEX; α-THIONEX®; β-THIONEX®; THIOKILL®; THIOFOR®; THIONEX®; THIOSULFAN®; THIOSULFAN THIONEL®; THISULFAN TIOVEL; TIONEL; TIOVEL®
Pharmacology
The rat LD50 values are 43, 18 mg/kg (oral) and 130,
74 mg/kg (dermal). The α-isomer has somewhat greater
insecticidal activity and is slowly converted to the more
stable β-isomer at a high temperature. Both isomers
oxidize slowly in air and in biological systems to endosulfan
sulfate [1031-07-8], mp 181–182 ?C. In acid media, both
isomers form endosulfan diol [2157-19-9], mp 203–205 ?C.
Endosulfan is a broad-spectrum insecticide used to control
pests of vegetables, fruit, field crops, and ornamentals.
Unlike other cyclodiene insecticides, it is biodegradable by
hydrolysis at the sulfite ester bonds and is more readily
metabolized. It is also less persistent on plant surfaces,
and 50% of the residues are lost in 3–7 days. Volatilization
may be the major route of loss.
Endosulfan is readily hydrolyzed in water to the diol
(74), but it is moderately persistent in soil. Endosulfan (α-
and β-endosulfan) is degraded in soil with DT50 30 to 70
days. The major metabolite is usually endosulfan sulfate
(71), which is degraded more slowly. In the field DT50
for total endosulfan (α- and β-endosulfan and endosulfan
sulfate) is 5 to 8 months.
Safety Profile
Poison by ingestion, inhalation, skin contact, intraperitoneal, and subcutaneous routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic and neoplastigenic data. Human systemic effects: convulsions, cyanosis. Human mutation data reported. A central nervous system stimulant producing convulsions. A htghly toxic organochlorine pesticide that does not accumulate significantly in human tissue. Absorption is normally slow, but is increased by alcohols, oil, and emulsifiers. When heated to decomposition it emits toxic fumes of Cl and SOx. See also CHLORIDES and SULFITES
Potential Exposure
Those engaged in the manufacture, formulation, and application of this material
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If this chemicalhas been inhaled, remove from exposure, begin rescuebreathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention.Consult hospital or poison control center on use of antidotes.Transport to health-care facility.
Carcinogenicity
Equivocal results have been found in genotoxic
assays, but endosulfan was mutagenic and
clastogenic and induced effects on cell cycle
kinetics in various in vivo and in vitro tests.
In reproductive studies, male rats treated
at 3.0 mg/kg from day 15 to 21 of gestation had
reduced sperm production in adulthood.
The 2003 ACGIH threshold limit
value-time-weighted average (TLV-TWA) is
0.1mg/m3 with a notation for skin absorption.
Metabolic pathway
When endosulfan is incubated with microorganisms, endosulfan is extensively degraded in nitrogen- deficient, carbon-deficient, and nitrogen-rich cultures of Phanerochaete chrysosporium and is primarily oxidized to endosulfan sulfate, which is a terminal end product, or hydrolyzed to the non-sulfur-containing metabolites. An initial hydrolysis of endosulfan results in the formation of the intermediate metabolite or endosulfan diol, which further undergoes oxidation to yield endosulfan hydroxyether followed by the formation of endosulfan lactone or tentatively identified endosulfan dialdehyde.
Metabolism
Endosulfan is metabolized rapidly inmammalian
organisms to less toxic metabolites and to polar
conjugates. The sulfate is also a majormetabolite in plants
and occurs as a metabolite in some mammals. Endosulfan
is quite toxic to water organisms, and residues were
found in runoff water, sediment, infiltration water, and
soil following a single application.
Rats dosed orally or intraperitoneally with endosulfan
(4–8 mg/Kg) excreted unchanged endosulfan, the hydroxy
ether (72), the lactone (73), and unidentified metabolites in
urine in the ratio 3 : 1 : 1 : 2. The diol, the hydroxy ether,
and the lactone were identified in most samples of urine
and feces. The metabolite most frequently recovered from
tissues, organs, and feces was endosulfan sulfate .
Transient amounts of endosulfanand endosulfan sulfate
were detected in the body fat and liver of mice after
they were dosed with 14C labeled endosulfan. The mice
excreted endosulfan metabolites. Cows fed 2.5–5 ppm
endosulfan for 30 days excreted 0.1–0.2 ppm endosulfan
sulfate in milk.
After a single dose of 14 mg/kg of 14C endosulfan, sheep
excreted 0.25 ppm in milk in the 6–24-h period following
ingestion (102). Residues fell to 0.04 ppm and 0.01 ppm
after 3 and 11 days, respectively. The main metabolites
in urine were the diol and the hydroxy ether.
Endosulfan sulfate, the ether, the hydroxy ether, the lactone,
and one unidentified metabolite were detected on the
surface when male migratory locusts (Pachytilus migratoides)
were exposed to endosulfan by oral, cutaneous, or
subcutaneous administration. Similarmetabolic pathways
were observed in the housefly and the cockroach. Six to
seven days after the last dose, neither endosulfan nor its
metabolites could be detected in locusts (103).
Endosulfan is very toxic to fish and caused many fish
deaths when the Rhine River became contaminated in
June 1969 (concentration was 0.1 ppm). The only residues
detected in fish exposed to acute and multiple subchronic
concentrations of endosulfan were endosulfan and the diol
and the glucuronic acid conjugate of the diol.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in a cool, dry, well-ventilated area, free ofalkalis, acids, and acid fumes. Where possible, automatically pump liquid from drums or other storage containers toprocess containers
Shipping
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Degradation
Endosulfan is slowly hydrolysed in aqueous acids and alkalies with the
formation of the diol(2) and sulfur dioxide.
Endosulfan is stable to sunlight but UV irradiation in solution gave a
number of products, the identity of which depended on the medium in
which photolysis took place (see Scheme 1) (Schumaker ef al., 1973). The
photolytic processes include reductive dechlorination and when the α-isomer
was irradiated in n-hexane, dechlorination took place at the double
bond to give 10, whereas in water-dioxane, it occurred at the methylene
bridge with the formation of 9. The products formed in the vapour phase
included the diol(2), the ether (4), the lactone (6), the cyclic sulfate (3), and
other products characterised as dechlorinated compounds (7 and 8).
The β-isomer of endosulfan gave compounds 11 and 12 in dioxane and
11 in n-hexane-acetone.
Toxicity evaluation
Endosulfan was not carcinogenic in the rat or mouse in chronic dietary studies. It was not genotoxic in a variety of tests. Kidney toxicity was observed in the rat at dietary levels of 100 ppm (5 mg/kg/day) and above. Clinical signs of hyperactivity and tremors were reported in many studies. No evidence was found of developmental or reproductive toxicity.
Incompatibilities
Those engaged in the manufacture, formulation, and application of this material
Waste Disposal
A recommended method for disposal is burial 18 in deep in noncropland, away from water supplies, but bags can be burned. Large quantities should be incinerated at high temperature in a unit with effluent gas scrubbing. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Thiosulfan Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | 1015@dideu.com | China | 2263 | 58 |
| changzhou huayang technology co., ltd | +8615250961469 | 2571773637@qq.com | China | 9821 | 58 |
| Hebei Duling International Trade Co. LTD | +8618032673083 | sales05@hbduling.cn | China | 15745 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
View Lastest Price from Thiosulfan manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-04-27 | Thiosulfan
115-29-7
|
US $0.00-0.00 / Kg | 1KG | 99.0% | 500 tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2019-07-06 | Thiosulfan
115-29-7
|
US $1.00 / kg | 1kg | 99% | Customized | Career Henan Chemical Co |
-

- Thiosulfan
115-29-7
- US $0.00-0.00 / Kg
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd
-

- Thiosulfan
115-29-7
- US $1.00 / kg
- 99%
- Career Henan Chemical Co
115-29-7(Thiosulfan)Related Search:
1of4