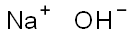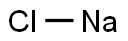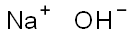Sodium hydroxide
- CAS No.
- 1310-73-2
- Chemical Name:
- Sodium hydroxide
- Synonyms
- NaOH;Caustic soda;Caustic Soda flakes;Caustic soda pearl;CAUSTIC FLAKES;Natriumhydroxid;IODINE SOLUTION;FLAKE CAUSTIC SODA;Hydroxyde de sodium;SODIUM HYDROXIDE, REAGENT GRADE, 97%, FL
- CBNumber:
- CB8105015
- Molecular Formula:
- NaOH
- Molecular Weight:
- 39.99711
- MDL Number:
- MFCD00011405
- MOL File:
- 1310-73-2.mol
- MSDS File:
- SDS
| Melting point | 681 °C(lit.) |
|---|---|
| Boiling point | 1390°C |
| Density | 1.515 g/mL at 20 °C |
| vapor density | <1 (vs air) |
| vapor pressure | 1 mm Hg ( 745 °C) |
| refractive index | 1,473-1,475 |
| Flash point | 176-178°C |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | beads |
| Specific Gravity | 2.13 |
| color | White |
| Odor | Odorless |
| PH Range | 13 - 14 |
| PH | 10.98(1 mM solution);11.95(10 mM solution);12.88(100 mM solution); |
| Water Solubility | SOLUBLE |
| Sensitive | Air Sensitive & Hygroscopic |
| Decomposition | 176-178 ºC |
| λmax |
λ: 260 nm Amax: 0.015 λ: 280 nm Amax: 0.01 |
| Merck | 14,8627 |
| Exposure limits | TLV-TWA air 2 mg/m3 (OSHA); ceiling 2 mg/m3 (ACGIH) and 2 mg/m3/15 min (NIOSH). |
| Dielectric constant | 57.5(25℃) |
| Stability | hygroscopic |
| CAS DataBase Reference | 1310-73-2(CAS DataBase Reference) |
| FDA 21 CFR | 184.1763; 582.1763; 173.310; 177.2800 |
| Substances Added to Food (formerly EAFUS) | SODIUM HYDROXIDE |
| SCOGS (Select Committee on GRAS Substances) | Sodium hydroxide |
| EWG's Food Scores | 1-4 |
| FDA UNII | 55X04QC32I |
| NIST Chemistry Reference | Sodium hydroxide(1310-73-2) |
| EPA Substance Registry System | Sodium hydroxide (1310-73-2) |
| Cosmetics Info | Sodium Hydroxide |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H314 | |||||||||
| Precautionary statements | P234-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 36/38-35-34 | |||||||||
| Safety Statements | 26-45-37/39-24/25-36/37/39 | |||||||||
| RIDADR | UN 1824 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TT2975000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2815 11 00 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD orally in rabbits: 500 mg/kg (10% soln) (Fazekas) | |||||||||
| IDLA | 10 mg/m3 | |||||||||
| NFPA 704 |
|
Sodium hydroxide price More Price(244)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SX0607N | Sodium hydroxide solution (10.0N) | 1310-73-2 | 1L | $76.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | SX0603 | Sodium Hydroxide Flakes Technical | 1310-73-2 | 12KG | $314 | 2024-03-01 | Buy |
| Sigma-Aldrich | 79724 | Sodium hydroxide solution 1?M, for HPCE | 1310-73-2 | 500ML | $355 | 2024-03-01 | Buy |
| Sigma-Aldrich | 567530 | Sodium Hydroxide, Pellets - CAS 1310-73-2 - Calbiochem | 1310-73-2 | 250g | $85.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 567530 | Sodium Hydroxide, Pellets - CAS 1310-73-2 - Calbiochem | 1310-73-2 | 500g | $102 | 2024-03-01 | Buy |
Sodium hydroxide Chemical Properties,Uses,Production
Chemical Properties
Sodium hydroxide is a white, odorless, nonvolatile alkaline material marketed in solid form as pellets, flakes, clumps, or sticks. Its solubility in water is 111% by weight and a vapor pressure of 0mmHg (NIOSH, 1994).
It can react with tricholoethylene (TCE) to form flammable dichloroacetylene and with metals to form hydrogen gas (OEHHA, 1993). Its reactivity with metals should be considered in regards to storage units and containers.

Sodium hydroxide is commonly available as an aqueous solution known as caustic soda, soda lye, or simple as lye. It has various uses, including neutralization of acid; the manufacture of paper, textiles, plastics, corrosives, dyestuffs, paint, paint remover, and soap; refining of petroleum; electroplating; metal cleaning; laundering; and dish washing. A burgeoning use has been in the illegal manufacture of methamphetamine.
Uses
Sodium hydroxide (NaOH) is one of the most useful industrial sodium compounds. It is also known as lye or caustic soda and is one of the strongest base alkalis (high pH value) on the household market. It is used as a drain and oven cleaner, and it saponifies fats in the manufacture of soap. It must be used with care because it is also capable of producing serious skin burns.
Description
Sodium hydroxide, also known as lye and caustic soda, is a highly caustic substance that is used used in small amounts in cosmetics to establish and hold the pH of a product.Sodium Hydroxide is a extremely important compound in our lives because it has so many uses. It is a very common base used in the chemical industry and is used for many things, many of which occur in our daily lives. One of the most well known uses of Sodium Hydroxide is its use in unclogging drains. It comes in many different brands of drain cleaners, but one of the most common is Drano. It also comes in the form of lye soap which can be used to wash practically anything, from the dishes to your face.
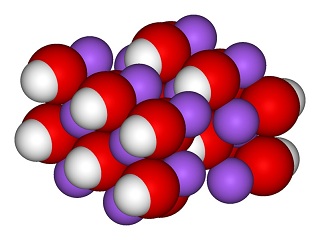
sodium hydroxide structure
At room temperature, sodium hydroxide is a white crystalline odorless solid that absorbs moisture from the air. It is a manufactured substance. Sodium Hydroxide is an inorganic compound used to control the pH levels or serve as a buffering agent in cosmetics and personal care products. It was historically used in the formulation of soaps, but is currently seen in a variety of formulas, including bath products, cleansing products, fragrances, foot powders, hair dyes and colors, makeup, nail products, personal cleanliness products, shampoos, shaving products, depilatories, skin care products, and suntan products, as well as chemical hair straighteners and hair wave sets. It is also a popular ingredient in industrial solvents as a chemical base for soaps, oven cleaners, detergents and drain cleaners because of its ability to dissolve grease, oils, fats and protein based deposits, according to Wikipedia. Less frequently, Sodium Hydroxide is seen as an ingredient in toothpastes.
Sodium Hydroxide is FDA approved, and has received the GRAS (Generally Recognized as Safe) rating as a direct food additive. However, it is primarily used in the washing and chemical peeling of produce. It is approved for use in cosmetics and personal care products in varying concentrations: 5% by weight in nail cuticle solvents, 2% by weight in hair straighteners for general use, 4.5% by weight in hair straighteners for professional use, up to a pH 12.7 in depilatories, and up to pH 11 in other uses as a pH adjuster.
References
https://pubchem.ncbi.nlm.nih.gov/compound/sodium_hydroxide
http://sodiumhydroxide.weebly.com/uses.html
Chemical Properties
Sodium hydroxide, NaOH,also referred to as caustic soda or sodium hydrate(and formerly known as lye), is a white,massive, deliquescent crystalline solid that is soluble in water,alcohol, and glycerol. It melts at 318°C (606 OF) and is the most widely used and available alkaline chemical. Most sodium hydroxide is produced as a coproduct of chlorine through the use of electrolytic cells;the cells are of the diaphragm, mercury, or membrane type. Some sodium hydroxide is marked as produced in the cells;most is evaporated and sold as 50% and 73% solutions or as anhydrous beads. Most caustic end uses require solutions of relatively low concentrations. Caustic soda is used as an analytical reagent and chemical intermediate, in scouring and cleaning baths,in rubber reclaiming and petroleum refining, in quenching baths for heat treating of steel,in cutting and soluble oils,in soaps and detergents, and in a wide variety of other applications.
Chemical Properties
NaOH is a white, odorless, deliquescent material sold as pellets, flakes, lumps, or sticks. Aqueous solutions are known as soda lye
Chemical Properties
Sodium hydroxide occurs as a white or nearly white fused mass. It is available in small pellets, flakes, sticks, and other shapes or forms. It is hard and brittle and shows a crystalline fracture. Sodium hydroxide is very deliquescent and on exposure to air it rapidly absorbs carbon dioxide and water.
Physical properties
White orthorhombic crystals, produced in the form of pellets, lumps, sticks, beads, chips, flakes or solutions; hygroscopic; very corrosive; rapidly absorbs CO2 and water from the air; density 2.13 g/cm3; melts at 323°C; vaporizes at 1388°C; vapor pressure 1 torr at 739°C and 5 torr at 843°C; very soluble in water (110 g/100mL at room temperature), generating heat on dissolution; aqueous solutions highly alkaline, pH of 0.5% solution about 13 and 0.05% solution about 12; soluble in methanol, ethanol and glycerol (23.8 g/100 mL methanol and 13.9 g/100 mL ethanol at ambient temperatures.).
Uses
Sodium Hydroxide is an alkali that is soluble in water, having a solubility of 1 g in 1 ml of water. it is used to destroy the bitter chemicals in olives that are to become black olives. it also functions to neutralize acids in various food products.
Uses
sodium hydroxide is used to adjust a product pH to make it more acceptable to the skin. It is commonly referred to as caustic soda, and often serves as a chemical reagent when making soap. If too concentrated it may cause severe skin irritation.
Uses
Caustic soda is one of the most widely usedchemicals. It is used to neutralize acids; tomake sodium salts; to precipitate metals astheir hydroxides; in petroleum refining; in thesaponification of esters; in the treatment ofcellulose, plastics, and rubber; and in numeroussynthetic and analytical applications.
Uses
Sodium hydroxide is sold commercially as anhydrous flakes or pellets or as 50% or 73% aqueous solutions. It has countless industrial uses and is one of the top 10 chemical in terms of production and use on a global scale. Approximately 15 million tons of sodium hydroxide is used annually. Its largest use, consuming about half of its production, is as a base in producing other chemicals. It is used to control pH and neutralize acids in chemical processes. The paper industry makes extensive use of sodium hydoxide in the pulping process. Sodium hydroxide is used to separate fibers by dissolving the connecting lignin. It is used in a similar fashion in the production of rayon from cellulose. Sodium hydroxide is a key chemical in the soap industry.In the saponification process, triglycerides obtained from animal and plants are heated in abasic solution to give glycerol and soap:
Sodium hydroxide is used in the textile industry for bleaching and treating textiles to makethem dye more readily. The petroleum industry uses sodium hydroxide in drilling muds and asa bactericide. Sodium hypochlorite (NaOCl) is used extensively for cleaning and as a disinfectant.Common household bleach consists of about 5% sodium hypochlorite solution. Sodiumhypochlorite is prepared by reacting chlorine with sodium hydroxide: Cl2(g) + 2NaOH(aq) →NaOCl(aq) + NaCl(aq) + H2O(l). Sodium hydroxide is used in the food industry for cleaningand peeling fruits and vegetables. Sodium hydroxide is a minor ingredient in many commonhousehold products, but in a few it may constitute more than half of the product. Dranocrystals contain between 30% and 60% sodium hydroxide and some drain cleaners can consistof 100% sodium hydroxide.
Uses
NaOH solutions are used to neutralize acids and make sodium salts, e.g., in petroleum refining to remove sulfuric and organic acids; to treat cellulose in making viscose rayon and cellophane; in reclaiming rubber to dissolve out the fabric; in making plastics to dissolve casein. NaOH solutions hydrolyze fats and form soaps; they precipitate alkaloids (bases) and most metals (as hydroxides) from water solutions of their salts. Pharmaceutic aid (alkalizer).
Uses
Sodium hydroxide is one of the most important industrial chemicals. In volume, it is in the top ten chemicals produced in the United States. It is used in manufacturing a large number of compounds including several sodium salts, in treating cellulose for producing rayon and cellophane, and in manufacturing soaps, detergents, pulp, and paper. Sodium hydroxide is a common neutralizing agent for acids in acid-base titrations and petroleum refining. Another major application is extracting metals from their ores where alkali fusion, such as fusion with caustic soda, often is applied to open the ores. Additionally, sodium hydroxide is used to precipitate metals as hydroxides. Other uses are in reclaiming rubber, dissolving casein in plastics production, refining vegetable oils, processing textiles, as an eluant in ion chromatography, etching and electroplating, and as a laboratory reagent. Sodium hydroxide also is used as a strong base in many organic synthesis and base-catalyzed reactions.
Preparation
Sodium hydroxide is manufactured together with chlorine by electrolysis of sodium chloride solution. Various types of electrolytic cells are used commercially. They include the mercury cell, the diaphragm cell, and the membrane cell.
A saturated solution of brine is electrolyzed. Chlorine gas is liberated at the anode and sodium ion at the cathode. Decomposition of water produces hydrogen and hydroxide ions. The hydroxide ion combines with sodium ion forming NaOH. The overall electrolytic reactions may be represented as:
2Na+ + 2Cl-+ 2H2O → Cl2 (g) + H2 (g) + 2NaOH (aq)
The mercury cell proceeds in two stages that occur separately in two cells. The first is known as the brine cell or the primary electrolyzer in which sodium ion deposits on the mercury cathode forming amalgam, while chlorine gas is liberated at the anode:
Na+ + Cl–→ Na-Hg (cathode) + ½Cl2(g) (anode)
In the second cell, known as the decomposer cell, a graphite cathode is used while sodium amalgam serves as the anode. Water reacts with the sodium metal of the amalgam in the decomposer:
Na-Hg + H2O → Na+ + OH– + ½H2↑ + Hg
In chlor-alkali diaphragm cells, a diaphragm is employed to separate chlorine liberated at the anode from the sodium hydroxide and hydrogen generated at the cathode. Without a diaphragm, the sodium hydroxide formed will combine with chlorine to form sodium hypochlorite and chlorate. In many cells, asbestos diaphragms are used for such separation. Many types of diaphragm cells are available.
Sodium hydroxide is produced either as an anhydrous solid or as a 50% aqueous solution.
Definition
The most important commercial caustic.
Production Methods
Sodium hydroxide is manufactured by electrolysis of brine using
inert electrodes. Chlorine is evolved as a gas at the anode and
hydrogen is evolved as a gas at the cathode. The removal of chloride
and hydrogen ions leaves sodium and hydroxide ions in solution.
The solution is dried to produce the solid sodium hydroxide.
A second method uses the Kellner–Solvay cell. Saturated sodium
chloride solution is electrolyzed between a carbon anode and a
flowing mercury cathode. In this case the sodium is produced at the
cathode rather than the hydrogen because of the readiness of
sodium to dissolve in the mercury. The sodium–mercury amalgam is
then exposed to water and a sodium hydroxide solution is
produced.
Reactions
Sodium hydroxide is strongly alkaline and can react with acids to form salts and water.
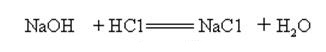
Sodium hydroxide reacts with acidic oxides to form salt and water, so sodium hydroxide can be used to absorb acid gases in the laboratory or industrially.
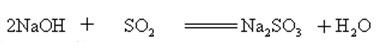
Sodium hydroxide can react with aqueous solutions of many metal salts to form sodium salts and metal hydroxides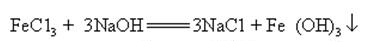
When sodium hydroxide and ammonia salt are heated together, it can release ammonia 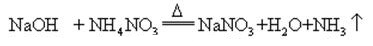
Sodium hydroxide is highly corrosive, so that the glass bottles storing sodium hydroxide solutions must be rubber stoppers, and glass stoppers should not be used to prevent a chemical reaction from opening. Sodium hydroxide is an important industrial raw material, and can be produced by electrolysis of saline solution industrially
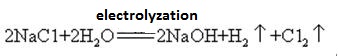
General Description
A white solid. Corrosive to metals and tissue. Used in chemical manufacturing, petroleum refining, cleaning compounds, drain cleaners.
Air & Water Reactions
Soluble in water. Dissolution can liberate enough heat to cause steaming and spattering and ignite adjacent combustible material [Haz. Chem. Data 1966].
Reactivity Profile
CAUSTIC SODA (Sodium hydroxide) is a strong base. Reacts rapidly and exothermically with acids, both organic and inorganic. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. Catalyzes the polymerization of acetaldehyde and other polymerizable compounds; these reactions can occur violently, for example, acrolein polymerizes with extreme violence when put in contact with alkaline materials such as sodium hydroxide [Chem. Safety Data Sheet SD-85 1961]. Reacts with great violence with phosphorus pentaoxide when initiated by local heating [Mellor 8 Supp.3:406 1971]. Contact (as a drying agent) with tetrahydrofuran, which often contains peroxides, may be hazardous---explosions have occurred in such a use of the chemically similar potassium hydroxide [NSC Newsletter Chem. Soc. 1967]. Mixing with any of the following substances in a closed container caused the temperature and pressure to increase: glacial acetic acid, acetic anhydride, acrolein, chlorohydrin, chlorosulfonic acid, ethylene cyanohydrin, glyoxal, hydrochloric acid (36%), hydrofluoric acid (48.7%), nitric acid (70%), oleum, propiolactone, sulfuric acid (96%) [NFPA 1991]. Accidental contact between a caustic cleaning solution (probably containing sodium hydroxide) and Pentol caused a violent explosion. [MCA Case History 363(1964)]. Heating with a mixture of methyl alcohol and trichlorobenzene during an attempted synthesis led to a sudden increase in pressure and an explosion [MCA Guide for Safety Appendix 3 1972]. Hot and/or concentrated NaOH can cause hydroquinone to decompose exothermically at elevated temperature. (NFPA Pub. 491M, 1975, 385)
Hazard
Corrosive to tissue in presence of mois- ture, strong irritant to tissue (eyes, skin, mucous membranes, and upper respiratory tract), poison by ingestion.
Health Hazard
Strong corrosive action on contacted tissues. INHALATION: dust may cause damage to upper respiratory tract and lung itself, producing from mild nose irritation to pneumonitis. INGESTION: severe damage to mucous membranes; severe scar formation or perforation may occur. EYE CONTACT: produces severe damage.
Health Hazard
Sodium hydroxide is a highly corrosive substancethat causes damage to human tissues.Its action on the skin is somewhat differentfrom acid burns. There is no immediate pain,but it penetrates the skin. It does not coagulateprotein to prevent its further penetration,and thus the caustic burn can become severeand slow healing. Spilling of its concentratedsolutions into the eyes can result in severeirritation or permanent injury.
It is toxic by ingestion as well as inhalationof its dust. Although the oral toxicity ofa 5–10% solution of caustic soda was foundto be low in test animals, high dosages atgreater concentrations can cause vomiting,prostration, and collapse. The oral lethal dosein rabbits is 500 mg/kg (NIOSH 1986).
Sodium hydroxide dusts or aerosols areirritating to the eyes, nose, and throat. Prolongedexposure to high concentrations in airmay produce ulceration of the nasal passage.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous solutions.
Pharmaceutical Applications
Sodium hydroxide is widely used in pharmaceutical formulations to adjust the pH of solutions. It can also be used to react with weak acids to form salts.
Industrial uses
Caustic soda (NaOH) is regarded as the strongest alkaline pH regulator. Caustic soda
is a very active substance and is highly corrosive. The bulk of caustic soda is manufactured
by electrolysis of saturated brines (NaCl). Caustic soda has a very strong pHregulating
capability (i.e. from pH 7 to pH 14) at a relatively low dosage compared to
other alkaline substances. Commercially, caustic soda is available in anhydrous form,
but in most mining applications the caustic soda is supplied as a 50% solution.
In the mineral processing industry, sodium hydroxide is mostly used for alkalinity control
during the processing of non-metallic minerals. In base metal flotation, the use of
sodium hydroxide is rare.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NanO.
Safety
Sodium hydroxide is widely used in the pharmaceutical and food
industries and is generally regarded as a nontoxic material at low
concentrations. At high concentrations it is a corrosive irritant to
the skin, eyes, and mucous membranes.
LD50 (mouse, IP): 0.04 g/kg
LD50 (rabbit, oral): 0.5 g/kg
Potential Exposure
NaOH is utilized to neutralize acids and make sodium salts in petroleum refining, viscose rayon; cellophane, plastic production; and in the reclamation of solutions of their salts. It is used in the manufacture of mercerized cotton, paper, explosives, and dyestuffs in metal cleaning; electrolytic extraction of zinc; tin plating; oxide coating; laundering, bleaching, dishwashing; and it is used in the chemical industries.
storage
Sodium hydroxide should be stored in an airtight nonmetallic container in a cool, dry place. When exposed to air, sodium hydroxide rapidly absorbs moisture and liquefies, but subsequently becomes solid again owing to absorption of carbon dioxide and formation of sodium carbonate.
storage
splash goggles and impermeable gloves should be worn at all times when handling these substances to prevent eye and skin contact. Operations with metal hydroxide solutions that have the potential to create aerosols should be conducted in a fume hood to prevent exposure by inhalation. NaOH and KOH generate considerable heat when dissolved in water; when mixing with water, always add caustics slowly to the water and stir continuously. Never add water in limited quantities to solid hydroxides. Containers of hydroxides should be stored in a cool, dry location, separated from acids and incompatible substances.
Shipping
UN1823 NaOH, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1824 NaOH, solution, Hazard class: 8; Labels: 8-Corrosive material
Purification Methods
Common impurities are water and sodium carbonate. Sodium hydroxide can be purified by dissolving 100g in 1L of pure EtOH, filtering the solution under vacuum through a fine sintered-glass disc to remove insoluble carbonates and halides. (This and subsequent operations should be performed in a dry, CO2-free box.) The solution is concentrated under vacuum, using mild heating, to give a thick slurry of the mono-alcoholate which is transferred to a coarse sintered-glass disc and evacuated free of mother liquor. After washing the crystals several times with purified alcohol to remove traces of water, they are dried in a vacuum, with mild heating, for about 30hours to decompose the alcoholate, leaving a fine white crystalline powder [Kelly & Snyder J Am Chem Soc 73 4114 1951]. CAUSTIC. Sodium hydroxide solutions (caustic), 14.77. Carbonate ion can be removed by passage through an anion-exchange column (such as Amberlite IRA-400; OH--form). The column should be freshly prepared from the chloride form by slow prior passage of sodium hydroxide solution until the effluent gives no test for chloride ions. After use, the column can be regenerated by washing with dilute HCl, then water. Similarly, other metal ions are removed when a 1M (or more dilute) NaOH solution is passed through a column of Dowex ion-exchange A-1 resin in its Na+-form. Alternatively, carbonate contamination can be reduced by rinsing sticks of NaOH (analytical reagent quality) rapidly with H2O, then dissolving in distilled H2O, or by preparing a concentrated aqueous solution of NaOH and drawing off the clear supernatant liquid. (Insoluble Na2CO3 is left behind.) Carbonate contamination can be reduced by adding a slight excess of concentrated BaCl2 or Ba(OH)2 to a NaOH solution, shaking well and allowing the BaCO3 precipitate to settle. If the presence of Ba in the solution is unacceptable, an electrolytic purification can be used. For example, sodium amalgam is prepared by the electrolysis of 3L of 30% NaOH with 500mL of pure mercury for cathode, and a platinum anode, passing 15 Faradays at 4Amps, in a thick-walled polyethylene bottle. The bottle is then fitted with inlet and outlet tubes, the spent solution being flushed out by CO2-free N2. The amalgam is then washed thoroughly with a large volume of deionised water (with the electrolysis current switched on to minimize loss of Na). Finally, a clean steel rod is placed in contact in the solution with the amalgam (to facilitate hydrogen evolution), reaction being allowed to proceed until a suitable concentration is reached, before being transferred to a storage vessel and diluted as required [Marsh & Stokes Aust J Chem 17 740 1964].
Incompatibilities
A strong base and a strong oxidizer. Violent reaction with acid. Incompatible with water; flammable liquids; organic halogens, nitromethane, and nitrocompounds, combustibles. Rapidly absorbs carbon dioxide and water from air. Contact with moisture or water may generate heat. Corrosive to metals. Contact with zinc, aluminum, tin and lead in the presence of moisture, forming explosive hydrogen gas. Attacks some forms of plastics, rubber or coatings.
Incompatibilities
Sodium hydroxide is a strong base and is incompatible with any compound that readily undergoes hydrolysis or oxidation. It will react with acids, esters, and ethers, especially in aqueous solution.
Waste Disposal
Discharge into tank containing water, neutralize, then flush to sewer with water.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental preparations; injections; inhalations; nasal, ophthalmic, oral, otic, rectal, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Sodium hydroxide Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| China Salt Changzhou Chemical Co., Ltd. | +86-519-88210708 +8613861226663 | postmaster@cz-chem.com | China | 1 | 58 |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | aroma@qdtrustagri.com | China | 165 | 58 |
| Nanjing Deda New Material Technology Ltd. | +8613223281135 | niki@njdeda.com | China | 76 | 58 |
| Chongqing Chuandong Chemical (Group) Co. Ltd. | +86-13637972665 +86-13637972665 | wxc@cd1958.com | China | 81 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 | catherine@yjchem.com.cn | China | 147 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 | sales8@anhuiyiao.com | China | 253 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| ARCTIC EXPORTS INC | +1-3026880818 +1-3026880818 | ARCTICEXPORTSINC@GMAIL.COM | Canada | 68 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
Related articles
- Sodium hydroxide:pH calculation,Uses,Health hazards
- Liquid sodium hydroxide is colorless and has no odor. It can react violently with strong acids and with water.
- Mar 6,2024
- Uses and safety of Sodium hydroxide
- Sodium hydroxide is sometimes called caustic soda or lye. It is a common ingrediet in cleaners and soaps.
- Jul 18,2022
- The review of sodium hydroxide
- Sodium hydroxide, also known as caustic soda, lye and caustic soda, with the chemical formula of NaOH, is a strong alkali with....
- Mar 17,2022
View Lastest Price from Sodium hydroxide manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Sodium hydroxide
1310-73-2
|
US $360.00 / ton | 50ton | 99% | 3000tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-26 | Sodium hydroxide
1310-73-2
|
US $544.00-535.00 / Tons | 1Tons | 99.99% | 100Tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-25 | Sodium hydroxide
1310-73-2
|
US $1.00 / g | 1g | 99 | 20tons | Shanghai Longyu Biotechnology Co., Ltd. |
-

- Sodium hydroxide
1310-73-2
- US $360.00 / ton
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Sodium hydroxide
1310-73-2
- US $544.00-535.00 / Tons
- 99.99%
- Hebei Dangtong Import and export Co LTD
-

- Sodium hydroxide
1310-73-2
- US $1.00 / g
- 99
- Shanghai Longyu Biotechnology Co., Ltd.