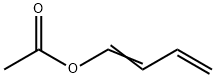
1-ACETOXY-1,3-BUTADIENE
- CAS No.
- 1515-76-0
- Chemical Name:
- 1-ACETOXY-1,3-BUTADIENE
- Synonyms
- 1-Acetoxybutadiene;Butadienyl acetate;1,3-BUTADIENYL ACETATE;1-ACETOXY-1,3-BUTADIENE;1,3-butadien-1-ol,acetate;1,3-BUTADIEN-1-YL ACETATE;Acetic acid-1,3-butadienyl;(E)-1,3-Butadienyl acetate;Buta-1,3-dien-1-yl acetate;(1E)-1,3-Butadienyl acetate
- CBNumber:
- CB6244909
- Molecular Formula:
- C6H8O2
- Molecular Weight:
- 112.13
- MDL Number:
- MFCD00008714
- MOL File:
- 1515-76-0.mol
| Boiling point | 60-61 °C40 mm Hg(lit.) |
|---|---|
| Density | 0.96 g/mL at 20 °C(lit.) |
| vapor pressure | 40 mm Hg ( 60 °C) |
| refractive index |
n |
| Flash point | 92 °F |
| storage temp. | 2-8°C |
| form | Liquid |
| BRN | 1743394 |
| CAS DataBase Reference | 1515-76-0(CAS DataBase Reference) |
| FDA UNII | N96404R1FH |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H226-H302-H311-H315-H319-H335 |
| Precautionary statements | P210-P233-P280-P301+P312-P303+P361+P353-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 10-21/22-36/37/38 |
| Safety Statements | 26-36/37 |
| RIDADR | UN 1992 3/PG 3 |
| WGK Germany | 3 |
| RTECS | EJ1225000 |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 2915390090 |
| Toxicity | skn-rbt 100 mg/24H open AIHAAP 23,95,62 |
1-ACETOXY-1,3-BUTADIENE price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 220868 | 1-Acetoxy-1,3-butadiene mixture of cis and trans | 1515-76-0 | 10g | $308 | 2024-03-01 | Buy |
| Usbiological | 408671 | 1-Acetoxy-1,3-butadiene | 1515-76-0 | 500mg | $355 | 2021-12-16 | Buy |
| TRC | A164285 | 1-Acetoxy-1,3-butadiene | 1515-76-0 | 1g | $120 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0006438 | 1-ACETOXY-1,3-BUTADIENE 95.00% | 1515-76-0 | 10G | $1232.15 | 2021-12-16 | Buy |
1-ACETOXY-1,3-BUTADIENE Chemical Properties,Uses,Production
Chemical Properties
clear colorless to slightly yellow viscous liquid
Uses
1-Acetoxy-1,3-butadiene was used as diene in the following reactions:
- Diels-Alder reaction with ortho-carbazolequinones to yield benzocarbazolequinone.
- Diels-Alder reaction with diethyl ketovinylphosphonate, with and without Lewis acid assistance.
- Diels-Alder reaction with methyl acrylate to yield racemic forms of 2-hydroxy-3-cyclohexenecarboxylic acid.
It was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate and during visible light photocatalysis. It was also used as reactant during intermolecular oxa-Pictet-Spengler cyclization.
General Description
1-Acetoxy-1,3-butadiene (1-ABD) can be generated by potassium or sodium acetate catalyzed reaction between crotonaldehyde and acetic anhydride. The product is a mixture of cis and trans forms, that has been confirmed by its physical properties and IR spectra. It is reported to be formed as a major product during the acetoxylation of 1,3-butadiene (BD) in gas-phase in the presence of Pd-KOAc (palladium-potassium acetate) catalyst. 1-ABD participates as 2Π or 4Π diene in cycloaddition reactions. Enantioselective Diels Alder reaction of 2-methoxy-5-methyl-1,4-benzoquinone with 1-ABD in the presence of molecular sieves (mesh size:4?) has been reported.
Safety Profile
Poison by inhalation. Moderatelytoxic by other routes. A skin irritant. Mutation datareported. When heated to decomposition it emits acridsmoke.
Purification Methods
The commercial sample is stabilised with 0.1% of p-tert-butylcatechol. If the material contains crotonaldehyde (by IR, used in its synthesis), it should be dissolved in Et2O, shaken with 40% aqueous sodium bisulfite, then 5% aqueous Na2CO3, water, dried (Na2SO4) and distilled several times in a vacuum through a Widmer [Helv Chim Acta 7 59 1924] (p 11) or Vigreux column (p 11) [Wicterle & Hudlicky Collect Czech Chem Commun 12 564 1947, Hagemeyer & Hull Ind Eng Chem 41 2920 1949]. [Beilstein 2 III 295.]
1-ACETOXY-1,3-BUTADIENE Preparation Products And Raw materials
Raw materials
Preparation Products
1-ACETOXY-1,3-BUTADIENE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 4006608290; 18621169109 | market03@meryer.com | China | 40241 | 62 |
| Alfa Aesar | 400-6106006 | saleschina@alfa-asia.com | China | 30132 | 84 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44751 | 61 |
| Shanghai Hanhong Scientific Co.,Ltd. | 021-54306202 13764082696; | info@hanhongsci.com | China | 42982 | 64 |
| Syntechem Co.,Ltd | info@syntechem.com | China | 12990 | 57 | |
| Shanghai T&W Pharmaceutical Co., Ltd. | +86 21 61551611 | China | 9901 | 58 |
1515-76-0(1-ACETOXY-1,3-BUTADIENE)Related Search:
1of4