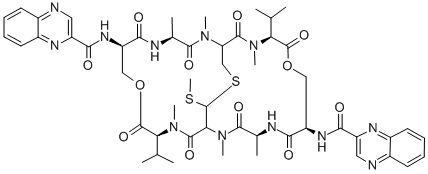
QUINOMYCIN A
- CAS No.
- 512-64-1
- Chemical Name:
- QUINOMYCIN A
- Synonyms
- SK-302B;nsc526417;NSC 13502;ECHINOMYCIN;echinomycina;QUINOMYCIN A;Echinomycin >97%;Antibiotic A 654I;Echinomycin (NSC-13502);EchinoMycin, Actinoleukin, 1491, 59266, X 948, X 53III
- CBNumber:
- CB6394145
- Molecular Formula:
- C51H64N12O12S2
- Molecular Weight:
- 1101.26
- MDL Number:
- MFCD00156105
- MOL File:
- 512-64-1.mol
- MSDS File:
- SDS
| Melting point | 217-218℃ |
|---|---|
| alpha | D20 -310° (c = 0.86 in chloroform) |
| Boiling point | 1427.2±65.0 °C(Predicted) |
| Density | 1.0964 (rough estimate) |
| refractive index | 1.6700 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 5 mg/ml) |
| pka | 9.38±0.70(Predicted) |
| form | White solid |
| color | white to beige |
| Merck | 13,3531 |
| Stability | Stable for 1 year as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months |
| EWG's Food Scores | 1 |
| FDA UNII | TG824J6RQT |
| NCI Drug Dictionary | echinomycin |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H361 | |||||||||
| Precautionary statements | P201-P202-P280-P301+P310-P302+P352+P312-P304+P340+P311 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 46-23/24/25 | |||||||||
| Safety Statements | 53-36/37/39-45 | |||||||||
| RIDADR | UN 3462 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | JW5250000 | |||||||||
| F | 10 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2941900000 | |||||||||
| NFPA 704 |
|
QUINOMYCIN A price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0477 | Echinomycin ≥98% (HPLC) | 512-64-1 | 1mg | $142 | 2024-03-01 | Buy |
| Cayman Chemical | 11049 | Echinomycin ≥98% | 512-64-1 | 1mg | $97 | 2024-03-01 | Buy |
| Cayman Chemical | 11049 | Echinomycin ≥98% | 512-64-1 | 5mg | $360 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0477 | Echinomycin ≥98% (HPLC) | 512-64-1 | 5mg | $588 | 2024-03-01 | Buy |
| Tocris | 5520 | Echinomycin ≥98%(HPLC) | 512-64-1 | 1 | $118 | 2021-12-16 | Buy |
QUINOMYCIN A Chemical Properties,Uses,Production
Description
Hypoxia-
Uses
Quinomycin A is a cyclic depsipeptide metabolite. Quinomycin A has broad activity against bacteria, fungi and viruses, and has found application as an antitumor agent. Quinomycin A acts by bifunctional intercalation of nucleic acids. Recent research has shown quinomycin A to be an extremely potent inhibitor of hypoxia-inducible factor-1 (HIF-1). This transcription factor plays an essential role in tumor progression and metastasis.
Uses
Quiniomycin A is a cyclic depsipeptide metabolite. Quinomycin A has broad activity against bacteria, fungi and viruses, and has found application as an antitumour agent. Quinomycin A acts by bifunctional intercalation of nucleic acids. Recent research has shown quinomycin A to be an extremely potent inhibitor of hypoxia-inducible factor-1 (HIF-1). This transcription factor plays an essential role in tumour progression and metastasis.
Uses
Echinomycin has been used for inhibition of hypoxia-inducible factor 1.
Biochem/physiol Actions
Echinomycin is an antitumor antibiotic and potent hypoxia inducible factor 1α (HIF-1α) inhibitor. It binds to DNA via bifunctional intercalation, blocking the binding of HIF-1α, a transcription factor important in tumor growth. Echinomycin selectively eliminated cancer stem cells in a study with mouse lymphoma and human AML xenogeneic models, eradicating the lymphomas. Recently, echinomycin was also found to act as an antibiotic adjuvant having synergistic effects with novobiocin in gram negative bacteria.
storage
Store at -20°C
References
1) Kong?et al.?(2005),?Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity; Cancer Res.,?65?9047 2) Wang?et al. (2014),?Echinomycin protects mice against relapsed acute myeloid leukemia without adverse effect on hematopoietic stem cells; Blood,?124?1127 3) Wang?et al. (2011),?Targeting HIF1α eliminates cancer stem cells in hematological malignancies; Cell Stem Cell.,?8?399 4) Li?et al. (2015),?Hypoxia-inducible factor-1α regulates the expression of L-type voltage-dependent Ca(2+) channels in PC12 cells under hypoxia; Cell Stress Chaperones,?20?507
QUINOMYCIN A Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | contact@fuxinpharm.com | China | 10297 | 58 |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | contact@trustwe.com | China | 5738 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Zhejiang Huida Biotech Co., LTD | 008613515763466 8615669048680 | wendy@huidabiotech.com | CHINA | 87 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-19164747840 +86-13119157289 | 13119157289@163.com | China | 2971 | 58 |