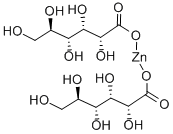
Zinc gluconate
- CAS No.
- 4468-02-4
- Chemical Name:
- Zinc gluconate
- Synonyms
- Zinc dihexonate;rubozine;Rubozinc;Aids002622;Aids-002622;Zinc complex;Givobio G Zn;Zinegluconate;Zinc gluconat;ZINC GLUCONATE
- CBNumber:
- CB6728315
- Molecular Formula:
- C12H22O14Zn
- Molecular Weight:
- 455.68
- MDL Number:
- MFCD00868110
- MOL File:
- 4468-02-4.mol
| Melting point | 172-175 °C |
|---|---|
| Boiling point | 319°C |
| Density | 1.44[at 20℃] |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Store at -20°C |
| solubility | Soluble in water, practically insoluble in anhydrous ethanol and in methylene chloride. |
| form | powder to crystal |
| color | White to Almost white |
| Water Solubility | Soluble in water 100g/ l. |
| Merck | 14,4456 |
| LogP | -7.41 at 20℃ |
| CAS DataBase Reference | 4468-02-4(CAS DataBase Reference) |
| FDA 21 CFR | 182.8988; 582.5988 |
| Substances Added to Food (formerly EAFUS) | ZINC GLUCONATE |
| SCOGS (Select Committee on GRAS Substances) | Zinc gluconate |
| EWG's Food Scores | 2-3 |
| FDA UNII | U6WSN5SQ1Z |
| NCI Drug Dictionary | zinc gluconate |
| ATC code | A12CB02 |
| EPA Substance Registry System | Zinc, bis(D-gluconato-.kappa.O1,.kappa.O2)-, (T-4)- (4468-02-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P501-P270-P264-P301+P312+P330 | |||||||||
| RTECS | ZH3750000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29181600 | |||||||||
| NFPA 704 |
|
Zinc gluconate price More Price(16)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TCI Chemical | G0277 | Zinc(II) Gluconate Hydrate >98.0%(T) | 4468-02-4 | 25g | $33 | 2024-03-01 | Buy |
| TCI Chemical | G0277 | Zinc(II) Gluconate Hydrate >98.0%(T) | 4468-02-4 | 500g | $135 | 2024-03-01 | Buy |
| Alfa Aesar | B23022 | Zinc gluconate hydrate, 97% | 4468-02-4 | 100g | $56 | 2024-03-01 | Buy |
| Alfa Aesar | B23022 | Zinc gluconate hydrate, 97% | 4468-02-4 | 500g | $151 | 2024-03-01 | Buy |
| TRC | Z701653 | ZincGluconateHydrate | 4468-02-4 | 250mg | $45 | 2021-12-16 | Buy |
Zinc gluconate Chemical Properties,Uses,Production
Organic zinc supplementation agent
Zinc can activate a variety of important antioxidant enzymes, thereby eliminating the damage of oxygen free radicals, maintaining the normal permeability of the cell membrane to protect the normal biochemical composition of cell membranes, metabolic structure and function. Zinc can not only induce the activation of T lymphocyte but also activate the B lymphocytes. Zinc is also involved in antibody formation and release, and stimulating immune cells to secrete a variety of cytokines. Lack of zinc in elderly people can cause immune function disorder; zinc can affect the synthesis of insulin, secretion, storage, degradation and biological activity, being the major trace amount element directly affecting the insulin physiology. Zinc can improve the body's sensitivity to insulin.
Zinc gluconate is an organic zinc supplement with fewer side effects than zinc sulfate and better absorption as well. It has certain efficacy on the treatment of zinc deficiency caused growth retardation, malnutrition, anorexia, recurrent oral ulcers, acne and senile zinc deficiency as well as immune dysfunction. It is mainly absorbed in the small intestine after oral administration with 1h reaching the peak but declining at about 2h. In the body, it is widely distributed in the liver, intestine, spleen, pancreas, heart, kidney, lung, muscle, central nervous system and bone. It is mainly excreted by the feces with a small amount excreted through the urine and milk. The bioavailability of it is about 1.6 times as high as that of zinc sulfate.
This product can be used to delay aging, enhance immune response, treatment of non-insulin-dependent diabetes. Oral administration amount calculated on zinc element is 10~25 mg 2 times per day and administrated after meals. Adverse reactions after meal mainly include stomach discomfort, nausea, vomiting. Zinc gluconate should not be subject to fasting medication and should not be overdosed which can affect the absorption of iron.
Uses
Zinc gluconate is an excellent nutrient zinc enhancer, having significant effect for the intellectual and physiological development of infants and young people with its absorption effect being better than inorganic zinc. China provides that it can be used for salt with the usage amount of 800~1000mg/kg; 230~470mg/kg in dairy product; 195~545mg/kg in infant and child food; in cereals and their products: 160~320mg/kg; 40 to 80 mg/kg in beverages and milk beverages.
Production method
There are many routes of synthesis of gluconate zinc with reasonable ways including fermentation, air catalytic oxidation and calcium gluconate indirect synthesis.
Fermentation
Take glucose as raw materials with Aspergillus fermentation (oxidation) to generate into gluconic acid. After separation, purification, gluconate is neutralized by zinc oxide or zinc hydroxide for preparation of zinc gluconate.
C6H12O6 + O2 [Aspergillus niger] → C6H11O6OH [ZnO] → (C6H11O6O) 2Zn + H2O
The method is mainly used domestically and abroad, the reaction is simple; easy to get raw materials; low cost, but the fermentation process requires a higher technology.
Air catalytic oxidation method
Under the presence of the catalyst, the glucose is oxidized by air to produce gluconic acid; and then the sodium hydroxide solution is added dropwise to until the pH value reaches 9.0 to 10.0 so as to be converted into sodium gluconate; after filtering and separating the catalyst, the sodium gluconate solution is oxidized by strong acid cations exchange resin into high-purity gluconic acid solution; finally reacted with zinc oxide or zinc hydroxide to form zinc gluconate with concentration, crystallization and re-crystallization to obtain the product with the yield being over 80%.
C6H12O6 + O2 [catalyst] → C6H11O6OH [NaOH] → C6H11O6ONa + H2O
C6H11O6Ona [R-H] → C6H11O6OH [ZnO] → (C6H11O6O) 2Zn + H2O
The method is easy to obtain raw materials with low cost and good product quality.
Indirect synthesis from calcium gluconate
Calcium gluconate, after being acidified by sulfuric acid is filtered to remove calcium sulfate precipitate to generate gluconic acid solution, which after being purified by ion exchange resin, has reaction with zinc oxide or zinc hydroxide to generate zinc gluconate; further carry out filtration, crystallization and drying to get the finished product.
(C6H11O6O) 2Ca [H2SO4] → C6H11O6OH [ZnO] → (C6H11O6O) 2Zn + H2O
Add 27 mL (0.5 mol) concentrated sulfuric acid to 500 mL water; successively add 224 g (0.5 mo1) of calcium gluconate and the reaction was carried out at 90 ° C for 1.5 h. The calcium sulfate precipitate was filtered off to give a pale yellow liquid. After cooling, the liquid flowed at a flow rate corresponding to resin volume per minute through the exchange column equipped with the same amount of anion and cation exchange resin to obtain a colorless transparent gluconic acid solution with the relative density of 1.10 to 1.12. The content is 20% and the yield is 95%.
Take 0.1 mol of gluconic acid solution, and separately add 4.1 g (0.05 mol) of zinc oxide for reaction at 60 ° C for 2 hours. Add drop wise of gluconate liquid to pH=5.7 at the same time and concentrate the 1/3 the volume of the original volume. Add 10 mL 95% ethanol and stand 8 h. The product was purified by crystallization, separation and vacuum drying to obtain 21.8 g product with a 96% yield and purity of 99.3%.
It can be obtained through the neutralization between zinc oxide and gluconic acid.
Identification test
The zinc test (IT-33) of the 10% sample liquid was positive.
Take 5 mL of 10% warm sample solution; add 0.7 ml acetic acid and 1 ml new distillated phenylhydrazine for heating in the steam bath for 30min, let it cool. Scratch the inner wall of container with glass rod and it should produce gluconic acid phenylhydrazine crystals.
Content analysis
Weigh approximately 700 mg of the sample, dissolve it in 100 ml of water (heat if necessary); add 5 ml of ammonia-ammonium chloride buffer solution (TS-12) and 0.1 ml of wool chromium black test solution (TS-97). Apply 0.05mol/L of EDTA disodium titration solution for titration to blue color. Each mL of 0.05 mL/L of EDTA disodium corresponds to 22.78 mg of zinc gluconate (C12H22O14Zn).
Usage limit
GB 14880-94 (mg/kg): dairy products 230~470; infant food 195~545; cereals and their products, beverages 80~160; salt: 500.
FDA, §182.5988 (2000): take GMP as limit.
GB 2760-2000: soft drinks 21.0~56mg/kg; children over one year old and children milk powder, 0.05~1.175g/kg (all in Zn).
According to the provisions of Japan, it is allowed to be used in breast milk substitute. The zinc content in per liter of standard milk concentration of breast milk substitute shall not exceed 6mg.
Chemical properties
It appears as white particles or crystalline powder with its molecule either containing or not containing three molecules of crystal water, being odorless and tasteless. It is easily soluble in water and slightly soluble in ethanol. Female mice by oral have a LD50 of 1.93g/k; male mice through oral have a LD50 of 2.99g/kg.
Toxicity
GRAS (FDA, § 182.8988, 2000); LD50 3.06 g/kg (mouse, oral);
Chemical Properties
White or almost white, hygroscopic, crystalline powder.
Uses
Chelating agent. In high alkalinity bottle washes and other cleansers; in finish removers; in the tanning and textile industry.
Uses
Zinc gluconate hydrate is used as a food additive, a pharmaceutical intermediate.
Uses
zinc gluconate helps maintain the skin in good condition and acts as a deodorant by preventing the growth of micro-organisms, such as bacteria, fungi or yeast, in a formulation. Zinc gluconate could also be effectively used in anti-acne products.
Definition
ChEBI: Zinc gluconate is a L-alpha-D-Hepp-(1->7)-L-alpha-D-Hepp-(1->3)-L-alpha-D-Hepp-(1->5)-alpha-Kdo.
Flammability and Explosibility
Not classified
Biotechnological Applications
Zinc gluconate is the most common standalone zinc supplement and is used in zinc lozenges, which are discussed later. In one study, the use of a zinc tolerance test shows zinc gluconate to be better absorbed than zinc sulfate. It can also be noted that zinc gluconate has given positive results in a number of supplementation studies, including some done for malnutrition situations. On the other hand, in a small unpublished study from this author, zinc gluconate was very ineffective at raising plasma zinc, while a zinc amino acid chelate raised plasma zinc in all subjects tested. In addition, in a rat supplementation study, neither zinc gluconate nor zinc sulfate was as effective as a zinc–amino acid chelate in protecting against chemically induced oxidant damage. Thus, zinc gluconate seems to be a good source of zinc for many purposes, though in some applications, it may not be the absolute best source.
Safety Profile
Experimental reproductive effects. W'hen heated to decomposition it emits toxic fumes of ZnO.
Zinc gluconate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 24099 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5882 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 10991 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 | admin@86-ss.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9357 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
Related articles
- Zinc Gluconate: Clinical Applications in Ulcerative Colitis and its Toxicity
- Zinc gluconate improves zinc levels and cytokines in ulcerative colitis, but intranasal use risks anosmia due to mucosal toxic....
- Aug 6,2024
View Lastest Price from Zinc gluconate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | Zinc gluconate
4468-02-4
|
US $990.00-800.00 / ton | 1ton | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-19 | Zinc Gluconate
4468-02-4
|
US $11.00 / KG | 1KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-18 | Zinc gluconate
4468-02-4
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- Zinc gluconate
4468-02-4
- US $990.00-800.00 / ton
- 99%
- HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
-

- Zinc Gluconate
4468-02-4
- US $11.00 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Zinc gluconate
4468-02-4
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD