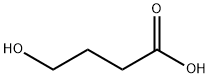
4-Hydroxybutyric acid
- CAS No.
- 591-81-1
- Chemical Name:
- 4-Hydroxybutyric acid
- Synonyms
- Butanoic acid, 4-hydroxy-;4-hydroxy-butanoic acid;4-HYDROXYBUTYRATE;4-Hydroxybutanoate;C00989;Anetamin;4-Hydroxybutyric;g-Hydroxybutyrate;4-Hydroxybutyric acid;GAMMA-HYDROXYBUTYRICACID
- CBNumber:
- CB7954553
- Molecular Formula:
- C4H8O3
- Molecular Weight:
- 104.1
- MDL Number:
- MOL File:
- 591-81-1.mol
| Melting point | 212℃ |
|---|---|
| Boiling point | 235.97°C (rough estimate) |
| Density | 1.1405 g/cm3 |
| refractive index | 1.4761 (estimate) |
| pka | 4.72(at 25℃) |
| EWG's Food Scores | 1 |
| FDA UNII | 30IW36W5B2 |
| EPA Substance Registry System | Butanoic acid, 4-hydroxy- (591-81-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H318-H302-H336 |
| Precautionary statements | P280-P305+P351+P338-P310-P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312-P403+P233-P405-P501 |
| Toxicity | LD50 oral in mouse: 4800mg/kg |
4-Hydroxybutyric acid Chemical Properties,Uses,Production
Description
γ-Hydroxybutyric acid (GHB), also known as 4- hydroxybutanoic acid, is a naturally occurring substance found in the human central nervous system, as well as in wine, beef, small citrus fruits, and almost all animals in small amounts. It is also categorized as an illegal drug in many countries. It is currently regulated in Australia and New Zealand, Canada, most of Europe and in the US. GHB as the sodium salt, known as sodium oxybate (INN) or by the trade name Xyrem, is used to treat cataplexy and excessive daytime sleepiness in patients with narcolepsy.
Uses
Anesthetic (intravenous). In treatment of narcolepsy; in treatment of alcoholism. This is a Schedule III controlled substance.
Definition
ChEBI: 4-Hydroxybutyric acid is a 4-hydroxy monocarboxylic acid that is butyric acid in which one of the hydrogens at position 4 is replaced by a hydroxy group.
brand name
Xyrem (Jazz).
Pharmacokinetics
4-Hydroxybutyric Acid (GHB) is found in all human tissues, with the highest concentration in the brain. This agent stimulates the GHB receptor, and to a lesser extent GABA-B receptors. Although, the precise function and metabolic pathways of GHB are not fully understood, this agent easily crosses the blood-brain barrier, and affects the activities and levels of dopamine, acetylcholine, dynorphin and serotonin. The primary effect of GHB is central nervous system depression, thereby, its main usage is to induce anesthesia.
Clinical Use
The only common medical applications for GHB today are in the treatment of narcolepsy and more rarely alcoholism.
GHB is the active ingredient in the prescription medication sodium oxybate (Xyrem). Sodium oxybate is approved by the U.S. Food and Drug Administration (FDA) for the treatment of cataplexy associated with narcolepsy and Excessive Daytime Sleepiness (EDS) associated with narcolepsy.
Metabolic pathway
Also note that both of the metabolic breakdown pathways shown for GHB can run in either direction, depending on the concentrations of the substances involved, so the body can make its own GHB either from GABA or from succinic semialdehyde. Under normal physiological conditions, the concentration of GHB in the body is rather low, and the pathways would run in the reverse direction to what is shown here to produce endogenous GHB. However, when GHB is consumed for recreational or health promotion purposes, its concentration in the body is much higher than normal, which changes the enzyme kinetics so that these pathways operate to metabolise GHB rather than producing it.
4-Hydroxybutyric acid Preparation Products And Raw materials
Raw materials
Preparation Products
591-81-1(4-Hydroxybutyric acid)Related Search:
1of4