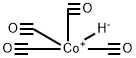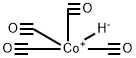Cobalt hydrocarbonyl.
- CAS No.
- 16842-03-8
- Chemical Name:
- Cobalt hydrocarbonyl.
- Synonyms
- Cobalt hydrocarbonyl.;Cobalt hydridotetracabonyl
- CBNumber:
- CB81246876
- Molecular Formula:
- C4HCoO4
- Molecular Weight:
- 171.98
- MDL Number:
- MOL File:
- 16842-03-8.mol
| form | Flammable gas with an offensive odor |
|---|---|
| Exposure limits |
TLV-TWA 0.1 mg/m3 as Co (ACGIH) PEL-TWA 0.1 mg/m3 as Co (OSHA). |
| FDA UNII | 33B757V1YU |
| EPA Substance Registry System | Cobalt hydrocarbonyl (16842-03-8) |
Cobalt hydrocarbonyl. Chemical Properties,Uses,Production
Description
Cobalt hydrocarbonyl is a highly flammableand toxic gas or liquid which decomposes rapidly at roomtemperature to toxic cobalt carbonyl. It has an offensiveodor. Molecular weight =171.98; Boiling point =10℃;Freezing/Melting point =-26℃; Flash point = flammablegas. Hazard Identification (based on NFPA-704 M RatingSystem): Health 2, Flammability 4, Reactivity 1. Veryslightly soluble in water.
Chemical Properties
Cobalt hydrocarbonyl is an unstable, highly flammable and toxic gas or liquid which decomposes rapidly at room temperature to toxic cobalt carbonyl. It has an offensive odor,
Uses
Catalyst in organic reactions
Uses
It is used as a catalyst in certain organicsynthesis.
Uses
Catalyst.
General Description
Gas with an offensive odor. Mp: -26°C
Air & Water Reactions
Air [Note: Unstable gas that decomposes rapidly in air at room temperature to cobalt carbonyl & hydrogen.]
Reactivity Profile
COBALT HYDROCARBONYL is flammable. Reacts exothermically with strong oxidizing agents and acids. Decomposes rapidly in air at room temperature to cobalt carbonyl (an orange solid of formula Co(CO)4) and hydrogen. These products also act as reducing agents. The orange solid (cobalt carbonyl) decomposes at 52°C producing toxic fumes of carbon monoxide and cobalt; reacts with acids and strong oxidizing agents below that temperature.
Hazard
Flammable gas. Possible carcinogen.
Health Hazard
The toxic effects are similar to those ofnickel tetracarbonyl or iron pentacarbonyl.The acute toxicity, however, is lower thanthat of these two carbonyls. Inhalation ofthe gas can cause dizziness, giddiness, andheadache. It readily decomposes at roomtemperature producing toxic carbon monoxide.A 30-minute LC50 in rats is 165 mg/m3(Palmes et al. 1959).
Fire Hazard
Flammable gas; the liquid form can explode when heated in a closed container due to rapid decomposition and heavy pressure buildup.
Safety Profile
Poison by inhalation. See also COBALT COMPOUNDS.
Potential Exposure
A potential danger to those involved in manufacture and use of this material as a catalyst for organic reactions.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 hours afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
Color Code—Yellow Stripe (strong reducingagent): Reactivity Hazard; Store separately in an area isolated from flammables, combustibles, or other yellow-codedmaterials. Prior to working with this chemical you shouldbe trained on its proper handling and storage. Store in acool, well-ventilated area. Procedures for the handling, use,and storage of cylinders should be in compliance withOSHA 1910.101 and 1910.169, as with the recommendations of the Compressed Gas Association.
Shipping
UN3281 Metal carbonyls, liquid, n.o.s., Hazard class: 6.1; Labels: 6.1 Technical Name Required, Potential Inhalation Hazard (Special Provision 5). Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner. UN2927 Toxic liquids, corrosive, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Technical Name Required.
Incompatibilities
Unstable gas; decomposes rapidly in air at room temperature to toxic cobalt carbonyl and explosive hydrogen gas. A strong metal hydride reducing agent; violent reaction with oxidizers and acids. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Aqueous solution is highly acidic. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides
Waste Disposal
Return refillable compressed gas cylinders to supplier.
Cobalt hydrocarbonyl. Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Portail Substances Chimiques | -- | webmestre-substances@ineris.fr | France | 6027 | 58 |
| SKC Inc. | -- | skcorder@skcinc.com | United States | 1379 | 76 |
| HONEST JOY HOLDINGS LIMITED | -- | sales@honestjoy.cn | United States | 6702 | 54 |
| Supplier | Advantage |
|---|---|
| Portail Substances Chimiques | 58 |
| SKC Inc. | 76 |
| HONEST JOY HOLDINGS LIMITED | 54 |
16842-03-8(Cobalt hydrocarbonyl.)Related Search:
1of2