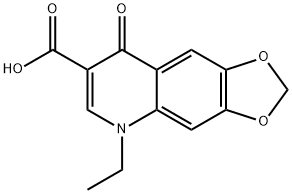
Oxolinic acid
- CAS No.
- 14698-29-4
- Chemical Name:
- Oxolinic acid
- Synonyms
- oxolinic;5-ETHYL-8-OXO-5,8-DIHYDRO-[1,3]DIOXOLO[4,5-G]QUINOLINE-7-CARBOXYLIC ACID;w4565;utibid;ossian;oxoboi;pietil;s-0208;uroxol;starner
- CBNumber:
- CB8177186
- Molecular Formula:
- C13H11NO5
- Molecular Weight:
- 261.23
- MDL Number:
- MFCD00056775
- MOL File:
- 14698-29-4.mol
| Melting point | 314-316°C (dec.) |
|---|---|
| Boiling point | 473℃ |
| Density | 1.3038 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| Flash point | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | Soluble in 0.5N NaOH with warming |
| form | Crystalline Powder |
| pka | 5.94±0.20(Predicted) |
| color | White |
| Water Solubility | 3.214mg/L(temperature not stated) |
| Sensitive | Light Sensitive |
| Merck | 13,7014 |
| BRN | 620635 |
| Stability | Stable. Combustible. |
| InChIKey | KYGZCKSPAKDVKC-UHFFFAOYSA-N |
| CAS DataBase Reference | 14698-29-4(CAS DataBase Reference) |
| FDA UNII | L0A22B22FT |
| ATC code | J01MB05 |
| EPA Substance Registry System | Oxolinic acid (14698-29-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302 | |||||||||
| Precautionary statements | P264-P270-P301+P312-P501 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22 | |||||||||
| Safety Statements | 22-24/25-60-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | JI5075000 | |||||||||
| F | 10 | |||||||||
| HS Code | 29349990 | |||||||||
| Toxicity | LD50 in mice, rats (mg/kg): >6000, >2000 orally (Turner) | |||||||||
| NFPA 704 |
|
Oxolinic acid price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | O0877 | Oxolinic acid quinolone antibiotic | 14698-29-4 | 5g | $138 | 2024-03-01 | Buy |
| Sigma-Aldrich | 67126 | Oxolinic acid analytical standard | 14698-29-4 | 100mg | $73.4 | 2024-03-01 | Buy |
| Alfa Aesar | J66637 | Oxolinic acid, 98% | 14698-29-4 | 5g | $170 | 2024-03-01 | Buy |
| Alfa Aesar | J66637 | Oxolinic acid, 98% | 14698-29-4 | 25g | $722 | 2024-03-01 | Buy |
| Cayman Chemical | 16789 | Oxolinic Acid ≥98% | 14698-29-4 | 1g | $32 | 2024-03-01 | Buy |
Oxolinic acid Chemical Properties,Uses,Production
Description
Developed in Japan in the 1970s, oxolinic acid belongs to the family of quinolone antibiotic, which is a synthetic antimicrobial agent aiming at gram-negative bacteria, especially those responsible for urinary tract infections. It is also commonly used in clinical for microbiological antimicrobial susceptibility tests against gram negative microbial isolates, providing antibiotic treatment options for infected patients. It can serve as a selective agent in several types of isolation media, for example, to isolate Gardnerella vaginalis. Besides, oxolinic acid can be applied in agriculture, which has proved to be effective against the seed-borne pathogen, such as Burkholderia glumae, a bacteria inducing grain rot, sheath rot, seedling rot, and bacterial panicle blight.
Oxolinic acid works by targeting DNA gyrase or topoisomerase II, enzymes vital for DNA synthesis, which ultimately inhibits DNA synthesis and cell division.
References
https://en.wikipedia.org/wiki/Oxolinic_acid
http://www.toku-e.com/product/oxolinic_acid/
https://www.goldbio.com/product/3027/oxolinic-acid
Description
Bacterial DNA gyrase is a heterodimeric type II topoisomerase that negatively supercoils circular double-
Chemical Properties
Crystalline Solid
Originator
Prodoxol,Warner,UK,1974
Uses
Quinolone antibacterial.
Uses
Oxolinic acid is a quinolone antibiotic used for studies on DNA winding and coiling and for studies on dopaminergic neurotransmission processes. It is used to study new transmissible resistance mechanisms qnrA, qnrB, qnrS, and aac(6′)Ib-cr, in Escherichia
Uses
Oxolinic acid is used to study new transmissible resistance mechanisms qnrA, qnrB, qnrS, and aac(6′)Ib-cr, in Escherichia coli and Salmonella enterica. Oxolinic acid is added to culture medium for the isolation of Gardnerella vaginalis.
Definition
ChEBI: A quinolinemonocarboxylic acid having the carboxy group at position 7 as well as oxo and ethyl groups at positions 4 and 1 respectively and a dioxolo ring fused at the 5- and 6-positions. A synthetic antibiotic, it is used in veterinary medicine for the tr atment of bacterial infections in cattle, pigs and poultry.
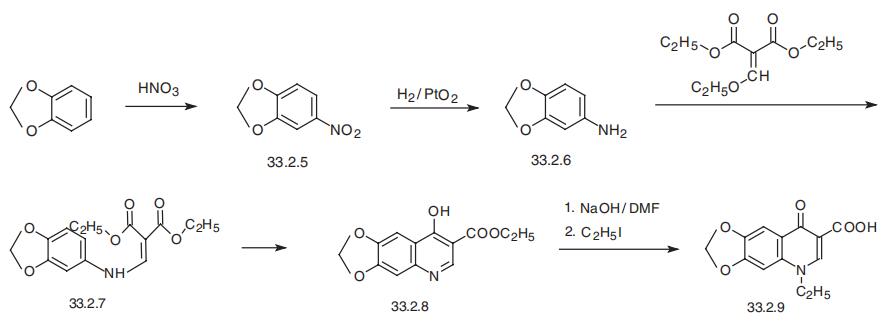
Manufacturing Process
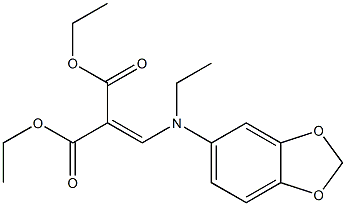
A mixture of 27 parts by weight of 3,4-methylenedioxyaniline and 43 parts by weight of diethyl ethoxymethylenemalonate is heated at 80° to 90°C for 3 hours. The mixture is then heated at 80° to 90°C for 1 hour under about 15 mm pressure to remove the byproduct ethyl alcohol formed. The residue is recrystallized from ligroin (BP 60° to 90°C) to give diethyl[(3,4methylenetlioxyanilino)methylene] malonate as a yellow solid melting at 100° to 102°C. The analytical sample from ligroin melts at 101° to 102°C.
A mixture of 48 parts by weight of diethyl[(3,4-methylenedioxyanilino) methylene] malonate and 500 parts by weight of diphenyl ether is refluxed for 1 hour. The mixture is allowed to cool to about 25°C with stirring and 500 parts by weight of petroleum ether are added. Filtration gives 3-carbethoxy6,7-methylenedioxy-4-hydroxy-quinoline as a brown solid, MP 276° to 281°C. Several recrystallizations from dimethylformamide gives almost colorless analytical material, MP 285° to 286°C, (decomposes).
A mixture of 26 parts of 3-carbethoxy-6,7-methylenedioxy-4-hydroxyquinoline,16 parts of sodium hydroxide and 50 parts of dimethylformamide is heated at 70° to 75°C for 2 hours, then 31 parts of ethyl iodide is added over 1 hour with continued heating and stirring. After an additional 3 to 4 hours of heating (at 70° to 75°C) and stirring, the mixture is diluted with 500 parts of water, refluxed for 3 to 4 hours, acidified with concentrated hydrochloric acid and filtered to yield 18 to 22 parts of 1-ethyl-1,4-dihydro-6,7-methylenedioxy-4-oxo-3-quinoline-carboxylic acid, MP 309° to 314°C (decomposes). The analytical sample from dimethylformamide melts at 314° to 316°C (decomposes).
brand name
Utibid (ParkeDavis).
Therapeutic Function
Antibacterial (urinary)
Antimicrobial activity
Like nalidixic acid, this drug is effective with respect to Gram-negative microorganisms and is used for the same indications. Synonyms of this drug are nidantin, prodoxol, ocolin, uroxol, and others.
General Description
Chemical structure: quinolone
Pharmaceutical Applications
An oral 4-quinolone with a tricyclic structure. Its antibacterial spectrum is very similar to that of nalidixic acid, but it is more active againstEnterobacteriaceae (MIC 0.25–2 mg/L). Grampositive bacteria, Ps. aeruginosa and anaerobes are resistant.
After repeated doses of 750 mg twice daily, mean plasma concentrations are initially very low, but steady state is reached at the third day and Cmax is around 3.5 mg/L. Administration with food delays absorption. Binding to plasma protein is about 80%. It undergoes complex biotransformation, and an enterohepatic cycle may account for the increase in the apparent elimination half-life from 4 to 15 h over 7 days of treatment as well as for the 20% of dose which can be recovered from the feces. About 50% of the dose appears in the urine in the first 24 h, partly in the form of metabolites, some of which display antibacterial activity.
Side effects common to the quinolones occur frequently. About one-quarter of patients treated with 750 mg every 12 h suffer nausea and vomiting or restlessness and insomnia. Its only use is in the treatment of lower urinary tract infections.
Biochem/physiol Actions
Oxolinic acid is a quinolone antibiotic. It is a DNA-gyrase (topoisomerase II) inhibitor used for studies on DNA winding and coiling and acts as a dopamine reuptake inhibitor for studies on dopaminergic neurotransmission processes.
Safety Profile
Moderately toxic by ingestion. Low toxicity by skin contact. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Oxolinic acid, 5-ethyl-5,8-dihydro-8-oxo-1-dioxolo[4,5-g]-quinolin- 7-carboxylic acid (33.2.9), is basically synthesized by the same synthetic scheme as nalidixic acid, although it uses 3,4-methylendioxyanyline (33.2.6) as the starting aromatic amine component, and not the 2-amino-6-methylpyridine used to make nalidixic acid. This compound is obtained by hydrogenation to 3,4-methylendioxy-1-nitrobenzene (33.2.5), which is in turn synthesized by nitrating 1,2-methylendioxybenzene with nitric acid. The resulting 3,4-methylendioxyaniline (33.2.6) is then reacted with ethoxymethylenmalonic ester to make the substitution product (33.2.7), which when heated cyclizes to ethyl ester of 4-hydroxy-6,7-methylendioxyquinolin-3-carboxylic acid (33.2.8). Hydrolyzis of this with a base in dimethylformamide and direct treating of the obtained product with ethyl iodide gives the desired oxolinic acid.
in vitro
previous study found that both inhibitors of dna gyrase of oxolinic acid and coumermycin a1 could block the dna synthesis in e. coli. moreover, the rate of bacterial dna synthesis first rapidly declined but then increased gradually at low concentrations of oxolinic acid. in varoius dna mutants, oxolinic acid was able to cause a rapid decline, followed by a slow decrease in synthesis rate of dna [1].
in vivo
animal study showed that the i.p. injection of oxolinic acid in mice could induce a dose dependent increase in locomotor activity, and such stimulation culminated at the 32 mg/kg dose and was smaller for higher doses at 64-128 mg/kg. when compared with haloperidol (d2 dopamine receptor antagonist) at increasing doses (50-100-200 mg/kg), the stimulant locomotor effect of oxolinic acid at 32 mg/kg was not reversed significantly. in addition, oxolinic acid at 32 mg/kg did not reverse the reserpine caused akinesia and even opposed the reversion that was induced by dexamphetamine [2].
IC 50
4.3 μm for dopamine uptake
Purification Methods
Purify the acid by recrystallisation from aqueous Me2CO, 95% EtOH or dimethylformamide. It has UV 220, (255.5sh), max 259.5, 268, (298sh, 311sh), 321 and 326nm [ 14.8, (36.8sh), 38.4, 38.4, (6.4sh, 9.2sh), 10.8 and 11.2 x 103]. [Kaminsky & Mettzer J Med Chem 11 160 1968, Beilstein 17 H 6, 17 I 3, 17 II 202, 17 III/IV 13, 17/1 V 11.]
References
[1] e c engle,s h manes, and k drlica. differential effects of antibiotics inhibiting gyrase. j bacteriol. 1982 jan; 149(1): 92–98.
[2] garcia de mateos-verchere j,vaugeois jm,naudin b,costentin j. behavioural and neurochemical evidence that the antimicrobial agent oxolinic acid is a dopamine uptake inhibitor. eur neuropsychopharmacol. 1998 dec;8(4):255-9.
Oxolinic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | aroma@qdtrustagri.com | China | 299 | 58 |
| Alfa Chemistry | Info@alfa-chemistry.com | United States | 24072 | 58 | |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12465 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5895 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Wuhan Haorong Biotechnology Co.,ltd | +86-18565342920; +8618565342920 | sales@chembj.net | China | 289 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1143 | 58 |
| Henan Suikang Pharmaceutical Co.,Ltd. | +86-18239973690 +86-18239973690 | sales@suikangpharm.com | China | 311 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 | admin@zlchemi.com | China | 3002 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1098 | 58 |
Related articles
- Uses and Toxicity of Oxolinic acid
- Oxolinic acid is a quinolone antibiotic developed in Japan in the 1970s.It also acts as a dopamine reuptake inhibitor and has ....
- Aug 23,2022
View Lastest Price from Oxolinic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | Oxolinic Acid
14698-29-4
|
US $20.70 / KG | 10KG | 99% | 10000kg | Hebei Chuanghai Biotechnology Co,.LTD | |
 |
2024-09-18 | Oxolinic acid
14698-29-4
|
US $0.00-0.00 / Kg/Drum | 1KG | 98%-102% | 20 tons | WUHAN FORTUNA CHEMICAL CO., LTD | |
 |
2024-09-09 | Oxolinic acid
14698-29-4
|
US $1.00-1.00 / kg | 1kg | 99% | 200000 | Wuhan Haorong Biotechnology Co.,Ltd |
-

- Oxolinic Acid
14698-29-4
- US $20.70 / KG
- 99%
- Hebei Chuanghai Biotechnology Co,.LTD
-

- Oxolinic acid
14698-29-4
- US $0.00-0.00 / Kg/Drum
- 98%-102%
- WUHAN FORTUNA CHEMICAL CO., LTD
-

- Oxolinic acid
14698-29-4
- US $1.00-1.00 / kg
- 99%
- Wuhan Haorong Biotechnology Co.,Ltd
14698-29-4(Oxolinic acid)Related Search:
1of4