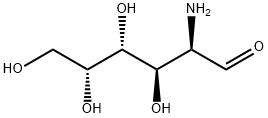
D-GALACTOSAMINE
- CAS No.
- 7535-00-4
- Chemical Name:
- D-GALACTOSAMINE
- Synonyms
- C02262;D-Chondrosamine;D-GALACTOSAMINE;d-2-amino-2-deoxygalactose;D-Galactose, 2-amino-2-deoxy-;(2R,3R,4R,5R)-2-Amino-3,4,5,6-tetrahydroxyhexanal
- CBNumber:
- CB8389318
- Molecular Formula:
- C6H13NO5
- Molecular Weight:
- 179.17
- MDL Number:
- MFCD01941613
- MOL File:
- 7535-00-4.mol
| Melting point | 185 °C |
|---|---|
| Boiling point | 532.5±50.0 °C(Predicted) |
| Density | 1.491±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| pka | 11.21±0.45(Predicted) |
| FDA UNII | 4Y6R29688W |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H319 |
| Precautionary statements | P280-P305+P351+P338 |
D-GALACTOSAMINE price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Matrix Scientific | 099675 | (2R,3R,4R,5R)-2-Amino-3,4,5,6-tetrahydroxyhexanal 95+% | 7535-00-4 | 250mg | $152 | 2021-12-16 | Buy |
| AK Scientific | 1707AC | (2R,3R,4R,5R)-2-Amino-3,4,5,6-tetrahydroxyhexanal | 7535-00-4 | 250mg | $255 | 2021-12-16 | Buy |
| Matrix Scientific | 099675 | (2R,3R,4R,5R)-2-Amino-3,4,5,6-tetrahydroxyhexanal 95+% | 7535-00-4 | 1g | $336 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CRB0001111 | D-GALACTOSAMINE 95.00% | 7535-00-4 | 1G | $405.3 | 2021-12-16 | Buy |
| AK Scientific | 1707AC | (2R,3R,4R,5R)-2-Amino-3,4,5,6-tetrahydroxyhexanal | 7535-00-4 | 1g | $503 | 2021-12-16 | Buy |
D-GALACTOSAMINE Chemical Properties,Uses,Production
Description
Galactosamine is a model hepatotoxicant, induces hepatitis
characterized by neutrophilic infiltration, and kills the animal
by fulminant hepatic failure. Galactosamine, an amino derivative
of sugar galactose, has been used as a model hepatotoxicant
since the first reports of hepatotoxicity in late 1970s by
Keppler and associates. Galactosamine-induced hepatitis has
been a model of choice to study various aspects of liver disease,
including mechanisms of toxicant-induced apoptosis and
necrosis, liver tissue repair, neutrophil infiltration and transmigration,
and the role of endotoxin or lipopolysaccharide
(LPS) in initiating liver injury.
Humans and animals synthesize galactosamine in the body.
Galactosamine (a type of hexosamine) is formed when an
amino group replaces one of the hydroxy groups of a sixcarbon
sugar, or hexose. The human body utilizes uridine
diphosphate (UDP)-N-acetyl-D-glucosamine or glucosamine as
a precursor to synthesize this compound, and is most often
found in the form N-acetyl-D-galactosamine (often referred to
as N-acetylgalactosamine). Most importantly, galactosamine is
a constituent of hyaluronic acid, a powerful water-binding
agent. Many different types of tissues in human body contain
hyaluronic acid, which acts as a lubricating agent in the synovial
fluid of joints and in connective tissues. Hyaluronic acid
also acts as a lubricating agent in the vitreous humor of the
eyeball. Hyaluronic acid has considerable medicinal value; it is
often used in wound healing, burn dressings, osteoarthritis
treatment, cataract or corneal transplantation surgery, and
various types of plastic surgeries.
Definition
ChEBI: The pyranose form of D-galactosamine.
Toxicity evaluation
Galactosamine induces liver injury by interfering with the uridine pool in the cell, which is essential for RNA and protein synthesis. Galactosamine ismetabolized via the Leloir pathway of galactose metabolism, which leads to the generation of uridine derivatives of galactosamine. The two enzymes of the Leloir pathway, galactokinase and UDP-galactose uridyltransferase, convert galactosamine into galactosamine-1-phosphate and UDP-galactosamine, respectively,due to their lowsubstrate specificity.UDPgalactosamine blocks the final enzyme in Leloir pathway, the UDP-galactose-40 epimerase, resulting in the accumulation of UDP-galactosamine in the cells. This results in the depletion of uridine triphosphate (UTP), UDP, uridine monophosphate (UMP), and the sugar derivative of uridine such as UDP-glucose and UDP-galactose essential for RNA and protein synthesis. Orotate, a precursor of the hexosamine biosynthesis pathway, has been used as an antidote to galactosamine toxicity.
D-GALACTOSAMINE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 7019 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-27-59207850 +86-13986145403 | info@fortunachem.com | China | 5989 | 58 |
| Aikon International Limited | 025-66113011 13155353615 | qzhang@aikonchem.com | China | 15396 | 58 |
| Jinan Kabotang Biological Technology Co.,Ltd. | 0531-61320525 15866703830 | 495745175@qq.com | China | 6891 | 58 |
| Shanghai Haohong Pharmaceutical Co., Ltd. | 400-400-8210725 4008210725 | malulu@leyan.com | China | 55023 | 58 |
| He' nan syndicate biology science and technology co., ltd | 17513242016 | 1576861551@qq.com | China | 1266 | 58 |
| Hubei Guangao Biotechnology Co., Ltd | 027-027-59223056 18162699093 | 1208480011@qq.com | China | 9951 | 58 |
| Hubei Wande Chemical Co., Ltd | 027-59210159 15377098680 | 1148280011@qq.com | China | 9994 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | 027-027-59207852 13308628970 | buy@fortunachem.com | China | 2640 | 58 |
View Lastest Price from D-GALACTOSAMINE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-01-11 | D-Galactosamine
7535-00-4
|
US $0.00 / KG | 1KG | 98%min | 30tons/month | WUHAN FORTUNA CHEMICAL CO., LTD |
-

- D-Galactosamine
7535-00-4
- US $0.00 / KG
- 98%min
- WUHAN FORTUNA CHEMICAL CO., LTD
7535-00-4(D-GALACTOSAMINE)Related Search:
1of4