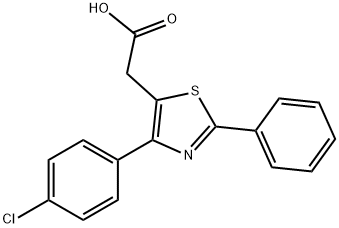
FENTIAZAC
- CAS No.
- 18046-21-4
- Chemical Name:
- FENTIAZAC
- Synonyms
- ch800;br700;flogene;donorest;norvedan;FENTIAZAC;4-(4-chlorophenyl)-2-phenyl-5-thiazoleaceticaci;4-(p-chlorophenyl)-2-phenyl-5-thiazoleaceticaci;4-(p-chlorophenyl)-2-phenylthiazole-5-aceticacid;4-(p-chlorophenyl)-2-phenyl-5-thiazoleaceticacid
- CBNumber:
- CB8736909
- Molecular Formula:
- C17H12ClNO2S
- Molecular Weight:
- 329.8
- MDL Number:
- MFCD00866039
- MOL File:
- 18046-21-4.mol
| Melting point | 162°C |
|---|---|
| Boiling point | 556.2±60.0 °C(Predicted) |
| Density | 1.1592 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| pka | 3.89±0.10(Predicted) |
| Water Solubility | 31.66mg/L(25 ºC) |
| FDA UNII | 0YHF6E6NLS |
| ATC code | M01AB10,M02AA14 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36/37/39 |
| Toxicity | LD50 in rats, mice (mg/kg): 661, 692 orally (Marmo) |
FENTIAZAC price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | API0002696 | FENTIAZAC 95.00% | 18046-21-4 | 10MG | $255.15 | 2021-12-16 | Buy |
| Crysdot | CD11215310 | 2-(4-(4-Chlorophenyl)-2-phenylthiazol-5-yl)aceticacid 95% | 18046-21-4 | 1g | $317 | 2021-12-16 | Buy |
| AHH | MT-51775 | Fentiazac 98% | 18046-21-4 | 0.1g | $350 | 2021-12-16 | Buy |
| Crysdot | CD11215310 | 2-(4-(4-Chlorophenyl)-2-phenylthiazol-5-yl)aceticacid 95% | 18046-21-4 | 5g | $624 | 2021-12-16 | Buy |
| Crysdot | CD11215310 | 2-(4-(4-Chlorophenyl)-2-phenylthiazol-5-yl)aceticacid 95% | 18046-21-4 | 10g | $942 | 2021-12-16 | Buy |
FENTIAZAC Chemical Properties,Uses,Production
Originator
Norvedan,LPB,Italy,1975
Uses
Anti-inflammatory.
Definition
ChEBI: 2-[4-(4-chlorophenyl)-2-phenyl-5-thiazolyl]acetic acid is a member of thiazoles.
Manufacturing Process
13.6 g methyl 3-(p-chlorobenzoyl)-3-bromopropionate in 30 ml methanol are
added to a solution of 5.6 g potassium thioacetate in 30 ml methanol.
Immediate precipitation of KBr is observed. The suspension is refluxed for 10
minutes.
It is cooled to ambient temperature, filtered, and the methanol is evaporated
to dryness. 13.2 g methyl 3-(p-chlorobenzoyl)-3-thioacetylpropionate in the
form of a chromatographically pure orange-colored oil are obtained.
A suspension of 13.2 g methyl 3-(p-chlorobenzoyl)-3-thioacetylpropionate is
agitated in 500 ml of a 2 N aqueous solution of KOH for 6 hours at ambient
temperature in an atmosphere of nitrogen, followed by extraction with ethyl
ether. The aqueous phase, adjusted to a pH equal to 2 with 2N HCl, is
extracted with ethyl ether which was washed with water, dried over Na2SO4,
and finally evaporated to dryness
9.8 g of crude 3-(p-chlorobenzoyl)-3-mercaptopropionic acid are obtained. By
recrystallizing from isopropyl ether there are obtained 8.6 g of pure product,
MP 96°C to 97°C (yield: 79%).
1.7 ml benzonitrile and 5.05 ml diethylamine are added to a solution of 4 g 3-
(p-chlorobenzoyl)-3-thiol-propionic acid in 50 ml ethanol. The solution is
agitated at ambient temperature for 60 minutes in an atmosphere of nitrogen.
It is then evaporated to a syrupy consistency and 60 ml 50% aqueous acetic
acid are added, whereupon the mixture is refluxed for 60 minutes. It is
evaporated to a small volume, adjusted to a pH equal to 8 with a saturated
solution of sodium bicarbonate and then extracted with ethyl ether. The
aqueous phase is acidified with 2N HCl (Congo red), and then again extracted
with ethyl ether. It is dried over Na2SO4 and evaporated to dryness. The
evaporation residue is recrystallized from benzene and 4 g 4-(p-chlorophenyl)-
2-phenyl-thiazol-5-yl-acetic acid are obtained (MP = 152°C to 154°C, yield -
74.3%).
Therapeutic Function
Analgesic, Antipyretic, Antiinflammatory
General Description
Fentiazac is reported to have anti-inflammatory, analgesic, and antipyretic activity. It has been given once or twice a day at levels between 100 and 200 mg/dose in the treatment of postoperative pain, including that following orthopedic surgery. The most common adverse effect is gastrointestinal intolerance, including epigastric pain, nausea, and vomiting. Effects on the CNS, such as headache and dizziness, also have been reported.
Trade name
Atilan (Zambon, Brazil), Donorest (Fontoura-Wyeth, Brazil), Flogene (Polifarma, Italy), Norvedan (Boehringer Mannheim, Austria; LPB, Italy).
Synthesis
Synthesis: refluxing a mixture of 3-bromo-
3-(4-chlorobenzoyl)propionic acid and thiobenzamide in ethanol gives ethyl 2-phenyl-4-(4-
chlorophenyl)thiazole-5-acetate, which is then
saponified to yield fentiazac.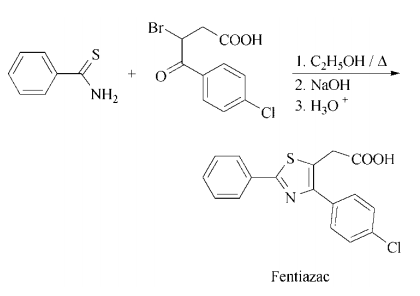
FENTIAZAC Preparation Products And Raw materials
Raw materials
Preparation Products
FENTIAZAC Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 | info@finetechnology-ind.com | China | 9633 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Finetech Industry Limited | 027-87465837 19945049750 | sales@finetechnology-ind.com | China | 9636 | 58 |
| Shanghai Haohong Pharmaceutical Co., Ltd. | 400-400-8210725 4008210725 | malulu@leyan.com | China | 55023 | 58 |
| Shaanxi Xihua Chemical Industry Co., Ltd | 17691182729 15529505138 | 1021@dideu.com | China | 10011 | 58 |