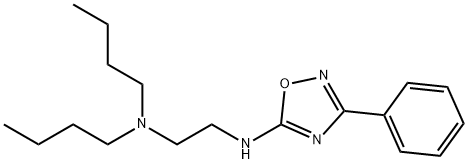
butalamine
- CAS No.
- 22131-35-7
- Chemical Name:
- butalamine
- Synonyms
- butalamine;Butalamina;3-Phenyl-5-(dibutylaMinoethylaMino)-1,2,4-oxadiazole;5-[2-(Dibutylamino)ethylamino]-3-phenyl-1,2,4-oxadiazole;N1,N1-Dibutyl-N2-(3-phenyl-1,2,4-oxadiazol-5-yl)-1,2-ethanediaMi;N,N-Dibutyl-N'-(3-phenyl-1,2,4-oxadiazol-5-yl)-1,2-ethanediaMine;1,2-Ethanediamine, N1,N1-dibutyl-N2-(3-phenyl-1,2,4-oxadiazol-5-yl)-
- CBNumber:
- CB8933148
- Molecular Formula:
- C18H28N4O
- Molecular Weight:
- 316.44
- MDL Number:
- MFCD00868274
- MOL File:
- 22131-35-7.mol
| storage temp. | Refrigerator |
|---|---|
| solubility | Chloroform )Slightly), Ethyl Acetate (Slightly) |
| form | Oil |
| color | Dark Brown |
| FDA UNII | 140T9JTG43 |
| ATC code | C04AX23 |
butalamine price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | B689930 | Butalamine | 22131-35-7 | 250mg | $1190 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0010890 | BUTALAMINE 95.00% | 22131-35-7 | 250MG | $1963.5 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB19369 | Butalamine | 22131-35-7 | 1mg | $70 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB19369 | Butalamine | 22131-35-7 | 2mg | $110 | 2021-12-16 | Buy |
| Biosynth Carbosynth | FB19369 | Butalamine | 22131-35-7 | 5mg | $190 | 2021-12-16 | Buy |
butalamine Chemical Properties,Uses,Production
Originator
Surheme,Aron,France,1969
Uses
Butalamine is a vasodilator (peripheral) with local anesthetic effects. Butalamine has been shown to inhibit state 3 respiration and decrease ADP/O in rat liver.
Definition
ChEBI: Butalamine is an oxadiazole and a ring assembly.
Manufacturing Process
Benzaldehyde and hydroxylamine may be reacted, the product chlorinated and
then reacted with cyanamide to give 5-amino-3-phenyl-1,2,4-oxadiazole.
32 grams of 3-phenyl-5-amino-1,2,4-oxadiazole dissolved in about 150 ml of
anhydrous benzene, 7.8 grams of sodium amide are added and the reaction
mixture heated at the boiling point with stirring for 2 hours. A solution of 38.3
grams of dibutylaminoethyl chloride in benzene is then added and the mixture
heated to boiling under reflux for four hours. The sodium chloride is separated
as previously described, the benzene removed by vacuum distillation and 56
grams of 3-phenyl-5-(dibutylaminoethylamino)-1,2,4-oxadiazole is obtained in
the form of an oil which is then converted directly to the crystalline
hydrochloride. This is accomplished by dissolving the oil in ethanol and adding
the stoichiometric equivalent of anhydrous ethyl ether saturated with gaseous
hydrogen chloride. The recrystallized salt is found to have a melting point of
145°C.
Therapeutic Function
Vasodilator
butalamine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 13028896684 | sales@rrkchem.com | China | 55954 | 58 |
| Changzhou Furuisi Biotechnology Co., Ltd | 0519-85524369 | 3477467573@qq.com | China | 8618 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24017 | 58 |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-63373950 15353716720 | 1052@dideu.com | China | 10011 | 58 |
| Carbosynth | -- | sales@carbosynth.com | United Kingdom | 6018 | 58 |