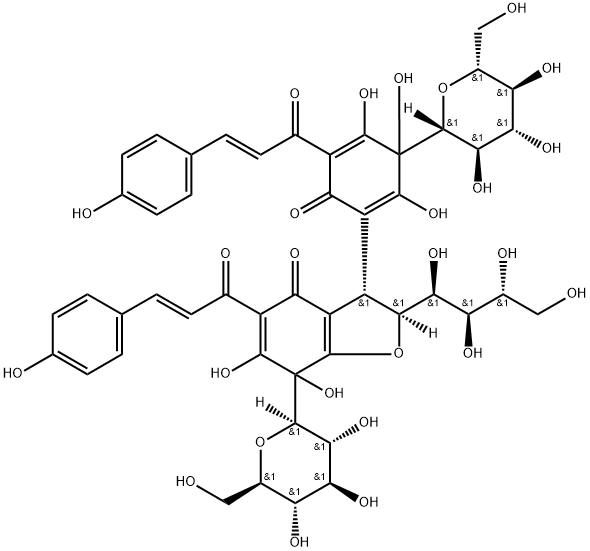
Anhydrosafflor yellow B
- CAS No.
- 184840-84-4
- Chemical Name:
- Anhydrosafflor yellow B
- Synonyms
- AHSYB;Anhydrosafflor yellow B;(2S,3S)-6,7-dihydroxy-5-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-2-[(1S,2R,3R)-1,2,3,4-tetrahydroxybutyl]-3-{2,3,4-trihydroxy-5-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-6-oxo-3-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]cyclohexa-1,4-dien-1;4(2H)-Benzofuranone, 7-β-D-glucopyranosyl-3-[3-β-D-glucopyranosyl-2,3,4-trihydroxy-5-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propenyl]-6-oxo-1,4-cyclohexadien-1-yl]-3,7-dihydro-6,7-dihydroxy-5-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propenyl]-2-[(1S,2R,3R)-1,2,3,4-tetrahydroxybutyl]-, (2S,3S)-
- CBNumber:
- CB93313458
- Molecular Formula:
- C48H52O26
- Molecular Weight:
- 1044.92
- MDL Number:
- MFCD32644781
- MOL File:
- 184840-84-4.mol
Anhydrosafflor yellow B Chemical Properties,Uses,Production
Description
Safflower, also known as red and blue flower, thorn safflower, mainly contains compounds such as chalcone pigments, flavonoids, phenolic acids, steroids and polysaccharides. Among the safflower yellows, hydroxyl safflower yellow A and dehydrated safflower yellow B (AHSYB) have the highest content, which are the main active ingredients with physiological activity in safflower.Anhydrosafflor yellow B can improve heart and brain blood oxygen supply, reduce ischemic injury, anticoagulant, anti-myocardial ischemia, inhibit platelet aggregation, anti-oxidation, anti-tumor and anti-inflammation and other pharmacological effects.
Biological Activity
Anhydrosafflor yellow B (AHSYB) is a quinolone C-glycoside isolated from Carthamus tinctorius. It inhibits ADP-induced platelet aggregation, has significant antioxidant effects in vitro, and has antitoxic activity against H2O2-induced PC12 cells and primary neuronal cells.
Synthesis
A method for extracting and purifying dehydrated safflower yellow B in safflower, 1) using warm soaking method to obtain safflower water extract; 2) pretreatment of macroporous adsorption resin; 3) macroporous resin adsorption; 4) macroporous resin Pore resin elution.
Anhydrosafflor yellow B Preparation Products And Raw materials
Raw materials
Preparation Products
Anhydrosafflor yellow B Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | sales@biopurify.com | China | 3424 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | marketing1@neostarunited.com | China | 8348 | 58 |
| Wuhan ChemNorm Biotech Co.,Ltd. | +86-27-8439 4403 18971486879 | sales@chemnorm.com | CHINA | 2935 | 58 |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | ada@ipurechemical.com | China | 10326 | 58 |
| HONG KONG IPURE BIOLOGY CO.,LIMITED | 86 18062405514 18062405514 | ada@ipurechemical.com | CHINA | 3465 | 58 |
| Hangzhou Huarong Pharm Co., Ltd. | 571-86758373 +8613588754946 | sales@huarongpharm.com | CHINA | 3149 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Shandong Xiya Chemical Co., Ltd | 13355009207 13355009207 | 3007715519@qq.com | China | 18739 | 57 |
| ShangHai YuanYe Biotechnology Co., Ltd. | 021-61312847 13636370518 | shyysw007@163.com | China | 4941 | 60 |
View Lastest Price from Anhydrosafflor yellow B manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-02-24 | Anhydrosafflor yellow B
184840-84-4
|
US $0.00 / mg | 5mg | ≥98%(HPLC) | 10 g | Shanghai Standard Technology Co., Ltd. |
-

- Anhydrosafflor yellow B
184840-84-4
- US $0.00 / mg
- ≥98%(HPLC)
- Shanghai Standard Technology Co., Ltd.