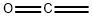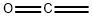ketene
- CAS No.
- 463-51-4
- Chemical Name:
- ketene
- Synonyms
- Keten;ethenone;carbometene;Ethen-1-one;Carbomethene;ketene ISO 9001:2015 REACH
- CBNumber:
- CB9934768
- Molecular Formula:
- C2H2O
- Molecular Weight:
- 42.04
- MDL Number:
- MOL File:
- 463-51-4.mol
- MSDS File:
- SDS
| Melting point | -150° |
|---|---|
| Boiling point | bp -56° |
| Density | 0.6600 |
| refractive index | 1.3040 (estimate) |
| form | Gas |
| Exposure limits | TLV-TWA 0.9 mg/m3 (0.5 ppm); STEL 3.0 mg/m3 (1.5 ppm) (ACGIH); IDLH 50 ppm (NIOSH). |
| FDA UNII | LEP3SM032A |
| EPA Substance Registry System | Ketene (463-51-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H315-H220-H330-H335-H318 |
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P280-P305+P351+P338-P310-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P210-P377-P381-P403 |
| RIDADR | 1955 |
| OEB | C |
| OEL | TWA: 0.5 ppm (0.9 mg/m3), STEL: 1.5 ppm (3 mg/m3) |
| HazardClass | 2.3 |
| IDLA | 5 ppm |
ketene Chemical Properties,Uses,Production
Description
Ketene is a flammable, colorless gas with asharp, disagreeable odor. Molecular weight = 42.04;Boiling point = 054.4℃; Freezing/Melting point =-148.3℃; Relative vapor density (air = 1): 1.45; Vaporpressure = <1 atm. Hazard Identification (based on NFPA-704 M Rating System): Health 4, Flammability 2,Reactivity 3. Reacts with water.
Chemical Properties
Colorless gas with a sharp penetrating odor;liquefies at -56°C (-68.8°F); solidifies at-151°C (-239.8°F); soluble in alcohol andacetone, decomposed by water.
Uses
For the conversion of higher acids into their anhydrides; for acetylation in the manufacture of cellulose acetate and aspirin.
Uses
Organic chemical syntheses; conversion of higher acids into their anhydrides; for acetylation in the manufacture of cellulose acetate and aspirin
Uses
Ketene is used as an acetylating agent inthe production of cellulose acetate, aspirin,acetic anhydride, and in various organicsyntheses.
Production Methods
Ketene may be prepared also by pyrolysis of acetic anhydride or phenyl acetate or diketene. Other sources are quite unsatisfactory from a standpoint of yield. Small quantities may be made conveniently by heating acetone in a “ketene lamp.” This is a glass apparatus containing a Nichrome filament, heated electrically to red heat. Larger amounts are made by passing acetone or acetic acid through a tube at 700 °C. A very brief contact time is required, so that much of the acetone is undecomposed and has to be condensed and recycled. Also, it is imperative that the reaction tube be of inert material such as porcelain, glass, quartz, copper or stainless steel. A copper tube, if used, should be protected from oxidation by an iron sheath. Inert packing may be used (glass, vanadium pentoxide, porcelain), but just as good yields are obtained with empty tubes. No catalyst is known which accelerates this decomposition at significantly lower temperatures.
Definition
ChEBI: Ethenone is a ketene.
Health Hazard
Ketene is a highly toxic gas. It causes severeirritation to the eyes, nose, throat, and skin.Exposure to 10–15 ppm for several minutescan injure the respiratory tract. It causespulmonary edema. A 30-minute exposure to23 ppm was lethal to mice and a 10-minuteexposure to 200 and 750 ppm caused deathto monkeys and cats.
Fire Hazard
Ketene in its gaseous state should be flammable and explosive in air. The pure compound, however, polymerizes readily and cannot be stored as a gas. Its flash point and LEL and UEL values are not reported. It can react violently with oxidizers and many organic compounds. Its small size and the olefinic unsaturation impart further reactivity to the molecule.
Potential Exposure
Ketene is used as an acetylating agentin cellulose acetate and aspirin manufacture; it is used inthe conversion of higher acids to their anhydrides; inthe production of spices, acetic anhydride, diethylaminogestrinone; ethyl acetate; ethyl acetoacetate; sorbic acid;vitamin A.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
Ketene cannot be stored. Ketene must be keptaway from water and a wide variety of organic compounds,since violent reactions occur. Ketene must be kept in tightlyclosed containers in a cool, well-ventilated area away fromheat. Metal containers involving the transfer of this chemical should be grounded and bonded. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical. Sources of ignition, such assmoking and open flames, are prohibited where this chemical is used, handled, or stored in a manner that could createa potential fire or explosion hazard. Wherever this chemicalis used, handled, manufactured, or stored, use explosionproof electrical equipment and fittings. Procedures for thehandling, use, and storage of cylinders should be in compliance with OSHA 1910.101 and 1910.169, as with therecommendations of the Compressed Gas Association.
Shipping
Ketene is not shipped as a rule, but is generated atthe point of use in a chemical process by acetone pyrolysis.It polymerizes readily and hence immediate use is preferred.
Purification Methods
Ketene is prepared by pyrolysis of acetic anhydride. Purify it by passing through a trap at -75o and collecting in a liquid-nitrogen-cooled trap. Ethylene is removed by evacuating the ethylene in an isopentane-liquid-nitrogen slush pack at -160o. Store it at room temperature in a suitable container in the dark or better at -80o, but do not store it under pressure as it may EXPLODE. It is a strong IRRITANT when inhaled and is as poisonous as phosgene. See diketene in “Heterocyclic Compounds”, Chapter 4. [Hurd Org Synth Coll Vol I 330 1941, Andreades & Carlson Org Synth Coll Vol V 679 1973.]
Incompatibilities
Forms an explosive mixture with air.Ketene readily polymerizes. Decomposes in water, alcohol,and ammonia. Reacts with water to form acetic acid.Contact with hydrogen peroxide forms explosive diacetylperoxide. Reacts violently with reducing agents, oxidizers,and many organic compounds. Can dimerize to diketeneeven at low temperatures. Diketene forms an explosive mixture with air.
ketene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Chizhou Kailong Import and Export Trade Co., Ltd. | xg01_gj@163.com | China | 9503 | 50 | |
| QUALITY CONTROL SOLUTIONS LTD. | 13670046396 | ORDERS@QCSRM.COM | China | 18899 | 58 |
| Hebei Dueling International Trade Co. LTD | 18032116176 | 399549586@qq.com | China | 1181 | 58 |
| Shandong Jiuyue Biological Technology Co., LTD | 15553348166 | m15553348166@163.com | China | 3524 | 58 |
| Dexin Chemical (Shandong) Co., LTD | 18560864701 | 957395144@qq.com | China | 3588 | 58 |
Related articles
- Ketene: Applications in Organic Synthesis and its Health Hazards
- Ketene is a versatile compound in organic synthesis but poses health risks, necessitating regulation in vaping products to saf....
- Apr 15,2024
- Production of Ketene
- Ketene is an extremely reactive and unstable compound. It is produced by pyrolysis of acetic acid [64-19-7], which, although e....
- Oct 21,2023