ペチジン 化学特性,用途語,生産方法
解説
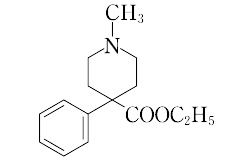
1-methyl-4-phenyl-4-piperidinecarboxylic acid ethyl ester.C15H21NO2(247.35).メペリジンともいう.ベンジルシアニドとN,N-ビス(2-クロロエチル)-N-メチルアミンから合成される.
"融点30 ℃,沸点155 ℃(666 Pa).偶然に発見された最初の合成麻薬である.おもに塩酸塩として使用される.塩酸塩は,融点187~189 ℃.水に易溶,エタノールに可溶,エーテルに不溶.モルヒネに似た鎮痛,鎮静作用を有し,より即効性であるが,効力は約1/10.かなり習慣性があり,慢性中毒によって大きな健康障害を起こす.アトロピン様の副交感神経作用とパパベリン様の鎮痙(けい)作用がある.LD50 170 mg/kg(ラット,経口).[CAS 57-42-1]
森北出版「化学辞典(第2版)
薬理学
ペチジンは,オピスタン,メペリジン,デメロールともいう。合成麻薬。鎮痛,鎮痙剤として使われる。モルヒネに似た鎮痛,鎮静作用のほかに副交感神経抑制作用,鎮痙作用を示すが,麻酔,鎮咳作用はほとんどない。おもに胃腸,胆嚢,胆管,尿管などの疼痛,骨折,関節痛などの疼痛,内視鏡検査や臨床検査時の疼痛緩和に用いられる。副作用としてはめまい,悪心,嘔吐,口渇,失神,恍惚感などがあり,大量では呼吸中枢抑制により死亡することもある。依存性があり,モルヒネより弱いが耐性,禁断症状も出現する。
効能
麻薬性鎮痛薬, オピオイド受容体作動薬
使用
Analgesic (narcotic).
生物学の機能
Meperidine (Demerol) is a phenylpiperidine derivative
of morphine that was developed in the late 1930s as a
potential anticholinergic agent. It has some anticholinergic
side effects that lead to tachycardia, blurred vision,
and dry mouth. Meperidine is approximately onefifth
as potent as morphine and is absorbed only half as
well when administered orally as parenterally. It has a
rapid onset and short duration of action (2 hours), that
is, approximately one-fourth that of morphine.
Like morphine, meperidine has an active metabolite,
normeperidine, formed by N-demethylation of
meperidine. Normeperidine is not analgesic but is a
proconvulsant and a hallucinogenic agent. For this reason,
meperidine use in patients with renal or liver insufficiency
is contraindicated because of the decreased
clearance of the drug and its metabolite. Convulsant activity
has been documented in elderly patients given
meperidine and in patients using PCA who have decreased
renal function.
Meperidine differs from morphine in that it has far
less antitussive effect and little constipative effect. The
drug is particularly useful in cancer patients and in pulmonary
patients, in whom the cough reflex must remain
intact. However, it does have more seizure-inducing activity
than morphine. Although meperidine produces
spasms of the biliary tract and colon, such spasms are of
shorter duration than those produced by morphine.
Meperidine readily passes the placenta into the fetus.
However, respiratory depression in the newborn
has not been observed, and meperidine clearance in the
newborn is rapid in that it does not rely upon conjugation
to glucuronides. Meperidine, unlike morphine, has
not been associated with prolongation of labor; conversely,
it increases uterine contractions.
一般的な説明
Meperidine (Demerol) was discovered in 1939 during a serendipitous screening of compounds being studied for antispasmodic activity. Mice given meperidine were noted to carry their tails in an erect position (the Straub tail reaction), which was indicative of narcotic analgesia. This led to the study of meperidine and derivatives as analgesic agents. Meperidine was found to have low potency at the receptor compared with morphine (0.2%) but much higher penetration into the brain resulting in a compound with about 10% of the potency of morphine.
Structural changes that increase the potency of meperidine include the introduction of an mhydroxyl on the phenyl ring, substituting the methyl on the N for a phenylethyl or a p-aminophenylethyl. Replacing the N-methyl with an N-allyl or N-cyclopropylmethyl group does not generate an antagonist, unlike the similar substitution of the morphine congeners. Meperidine quickly penetrates the blood-brain barrier and thus has a quick onset of activity and a high abuse potential.
予防処置
Contraindications are similar to those of morphine. In
addition, because normeperidine accumulates in renal
dysfunction and meperidine accumulates in hepatic dysfunction,
meperidine is contraindicated in such patients
because of convulsant effects. Similarly, the use of
meperidine is contraindicated in patients who have a history of seizures or who are taking medication to prevent
seizures. Phenytoin administered for seizures may
reduce the effectiveness of meperidine by increasing
the metabolism of the drug in the liver. Meperidine is
not generally used in patients with cardiac dysfunction,
since its anticholinergic effects can increase both heart
rate and ectopic beats.
ペチジン 上流と下流の製品情報
原材料
準備製品