ベリリウムジクロリド 化学特性,用途語,生産方法
解説
塩化ベリリウム.酸化ベリリウムと炭素の混合物を900 ℃ に加熱して塩素ガスを通じると無水物が得られる.無色の単斜晶系結晶.密度1.89 g cm-3.融点440 ℃,沸点547 ℃.熱を発生して水に易溶.水溶液から四水和物が得られる.エタノール,エーテルにも可溶.重合しやすく,気体中でも一部はBe2Cl4となっている.AlCl3に似た点が多く,フリーデル-クラフツ反応の触媒となる.融解塩電解によるベリリウム金属製造の原料として用いられる.有毒.アルコール,ベンゼン,エーテル,二硫化炭素に可溶。4水和物 BeCl2・4H2O は単斜晶系,潮解性の板状晶。ベリリウム金属の製造に用いられる。体中に入ると猛毒。
化学的特性
Beryllium chloride is available as colorless to yellow crystals. It decomposes rapidly on
contact with water producing hydrogen chloride, and attacks many metals in the presence
of water. Beryllium chloride emits irritating or toxic fumes (or gases) in a fi re.
物理的性質
Beryllium chloride has the formula BeCl2 with a molecular weight of 79.9176 g/mol. Beryllium chloride, BeCl2, melts at 405°C and boils at 520°C. That compares with 714°C and 1412°C for magnesium chloride. The solid is a one-dimensional polymer consisting of edge-shared tetrahedra. In contrast, BeF2 is a threedimensional polymer, with a structure akin to that of quartz. In the gas phase, BeCl2 exists both as a linear monomer and a bridged dimer with two bridging chlorine atoms where the beryllium atom is 3-coordinate. This linear shape contrasts with the monomeric forms of some of the dihalides of the heavier members of group 2, e.g. CaF2, SrF2, BaF2, SrCl2, BaCl2 and Ba that are all nonlinear.
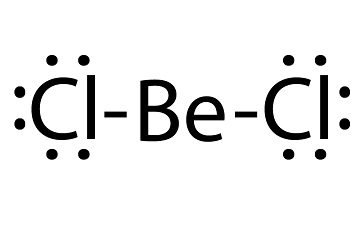
beryllium chloride lewis structure
The pure chloride is a glassy, transparent mass having a specific gravity of 2.01 at 15°C. It becomes fluid toward 440°C, passing through a viscous condition, but above 800° C it begins to volatilize, yielding white and very deliquescent crystals. It dissolves readily in water, but is only slightly soluble in absolute alcohol. By cooling an alcoholic solution to 23°C, one can obtain a white crystalline mass which, however, melts easily as the temperature rises.
Beryllium chloride, an electron-deficient compound similar to aluminum chloride, is a Lewis acid. The anhydrous salt is used as a catalyst in organic reactions.
来歴
Although early workers (1798) obtained the chloride
in solution, the pure salt was not made until about
1827 when one worker of the time prepared it in the
sublimed anhydrous state by passing chlorine gas over
a heated mixture of carbon and beryllium oxide. Finally
(1885), it was prepared as the pure chloride in very pure
form for the purpose of determining its vapor density by
the action of dry hydrochloric acid gas on the metal, but
the pure salt was not produced until 1898 when one
worker made it by heating the double fluoride of ammonium
and beryllium, which had previously been dried
over phosphoric anhydride, in a current of dry carbon
dioxide and cooled in an atmosphere of the same gas.
He also prepared it by the action of hydrofluoric acid
gas on the carbide. No matter what method is used, it
was determined that the materials must be absolutely
dry if a pure chloride is to be obtained.
使用
Beryllium chloride (BeCl2) is used as a catalyst to accelerate many organic reactions, and
beryllium chloride is the electrolyte used along with NaCl in the electrolytic process to produce
beryllium metal.
製造方法
Beryllium chloride is prepared by passing chlorine over beryllium oxide and carbon:
BeO + C + Cl
2 → BeCl
2 + CO
It also is made by combination of beryllium with chlorine.
定義
ChEBI: A compound of beryllium (+2 oxidation state) and chloride in the ratio 1:2.
一般的な説明
White to green solid with a sharp odor.
空気と水の反応
Reacts with water with evolution of heat. Forms beryllium oxide and hydrochloric acid solution. Corrodes most metals in presence of moisture. Flammable and explosive hydrogen gas may collect in enclosed spaces [USCG, 1999].
反応プロフィール
Acidic salts, such as BERYLLIUM CHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions. BERYLLIUM CHLORIDE reacts with vigor with sulfur nitrides. Some explode upon intimate mixing, i.e. tetrasulfur tetranitride.
危険性
Very toxic.
健康ハザード
Exposures to beryllium chloride cause redness, pain and blurred vision, nausea, vomiting,
and abdominal pain. Inhalation of beryllium chloride causes cough, sore throat, shortness
of breath
火災危険
Special Hazards of Combustion Products: Toxic and irritating beryllium oxide fumes and hydrogen chloride may form in fires.
安全性プロファイル
Confirmed carcinogen withexperimental tumorigenic data. Poison by ingestion andintraperitoneal routes. An experimental teratogen. Otherexperimental reproductive effects. Mutation data reported.When heated to decomposition it emits very toxic fumesof
合成
Beryllium chloride can be prepared directly from beryl by chloride or by chlorination of beryllium oxide under reducing conditions. Beryllium chloride is especially well suited for purification by distillation in a stream of hydrogen and fractional condensation. The significantly lower-boiling chlorides of aluminum, silicon, and iron(III) can be separated by careful temperature control. Iron(II) chloride, which is reduced by hydrogen, stays in the residue.
ベリリウムジクロリド 上流と下流の製品情報
原材料
準備製品