コルヒチン 化学特性,用途語,生産方法
外観
白色~わずかにうすい黄色, 結晶性粉末~粉末
溶解性
水に微溶, エタノールに易溶, エーテルに難溶。クロロホルムに可溶、石油エーテルに不溶。エタノールに溶けやすく、水にやや溶けにくく、アセトンに極めて溶けにくい。
解説
C22H25NO6(399.44).ユリ科イヌサフランColchicum autumnaleの種子に多く含まれている(約0.8%),トロポロン核をもった中性アルカロイド.
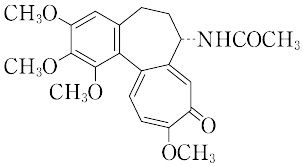
"黄色の結晶.融点155 ℃.
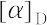
"-121°(クロロホルム).低濃度で植物の染色体倍加作用を有し,4倍体を得ることができるので貴重な薬品である.痛風発作の緩解や予防に有効であるが,中枢神経の麻ひ作用を有し,大量では呼吸麻ひによって死に至る.LD50 1.7 mg/kg(マウス,静注).[CAS 64-86-8]
森北出版「化学辞典(第2版)
用途
チューブリンに特異的に結合 することによって、微小管形成阻害作用を示 します。
用途
チューブリンに特異的に結合
することによって、微小管形成阻害作用を示
します。
用途
同調培養用試薬。
用途
コルヒチン(colchicine)とはイヌサフラン科のイヌサフラン(Colchicum autumnale)の種子や球根に含まれるアルカロイドである。リウマチや痛風の治療に用いられてきたが、毒性も強く下痢や嘔吐などの副作用を伴う。また種なしスイカなどの倍数体植物種の作出にも用いられる。
用途
コルヒチンは,アルカロイドの一種で、ユリ科のイヌサフラン(コルチカム)の種子や球茎に含まれる。分子式はC22H25NO6で、炭素の7員環をもった構造である。分裂中の植物細胞に作用して紡錘体の形成を阻害し、染色体の倍化をおこすので、細胞遺伝学の研究や育種に利用されている。種なしスイカなどの品種改良はその例である。医薬としては急性の痛風発作の特効的治療薬として知られる。発作の起り始め,とくに数時間以内に治療を開始すれば大部分の患者は激烈な痛みから救われる。しかしこの薬物は痛風以外の関節炎,関節リウマチなどには無効であり,また鎮痛作用も認められない, 長期連用により血液障害や脱毛、発疹(はっしん)、胃腸障害などの副作用がみられる。1錠中に0.5ミリグラム含有、1日3~4ミリグラムを6~8回に分けて服用する。[幸保文治・星川清親]
効能
痛風治療薬, 白血球(好中球)遊走阻害薬, 微小管重合阻害薬
商品名
コルヒチン (高田製薬)
確認試験
(1) 本品のエタノール(95)溶液(1→100000)につき,紫外
可視吸光度測定法〈2.24〉により吸収スペクトルを測定し,
本品のスペクトルと本品の参照スペクトルを比較するとき,
両者のスペクトルは同一波長のところに同様の強度の吸収を
認める.
(2) 本品のメタノール溶液(1→50)0.5mLを赤外吸収スペ
クトル用臭化カリウム1gに加え,よくすり混ぜた後,80℃
で1時間減圧乾燥したものにつき,赤外吸収スペクトル測定
法〈2.25〉の臭化カリウム錠剤法により試験を行い,本品の
スペクトルと本品の参照スペクトルを比較するとき,両者の
スペクトルは同一波数のところに同様の強度の吸収を認める.
定量法
本品約0.4gを精密に量り,無水酢酸25mLに溶かし,
0.05mol/L過塩素酸で滴定〈2.50〉する(電位差滴定法).同様
の方法で空試験を行い,補正する.
純度試験
5mLに塩化鉄(Ⅲ)試液2滴を加えるとき,液は明らかに認め
られる緑色を帯びない.
(2) 酢酸エチル及びクロロホルム 本品約0.6gを精密に量
り,内標準溶液2mLを正確に加えて溶かし,更にN,N-ジ
メチルホルムアミドを加えて10mLとし,試料溶液とする.
別にN,N-ジメチルホルムアミド約20mLを入れた100mLの
メスフラスコを用い,クロロホルム0.30gを量り,N,N-ジ
メチルホルムアミドを加えて正確に100mLとする.この液
2mLを正確に量り,N,N-ジメチルホルムアミドを加えて
正確に200mLとし,標準溶液(1)とする.次にN,N-ジメチ
ルホルムアミド約20mLを入れた100mLのメスフラスコを用
い,酢酸エチル約1.8gを精密に量り,N,N-ジメチルホルム
アミドを加えて正確に100mLとする.この液2mLを正確に
量り,内標準溶液2mLを正確に加え,N,N-ジメチルホル
ムアミドを加えて10mLとし,標準溶液(2)とする.試料溶液,
標準溶液(1)及び標準溶液(2)2μLずつを正確にとり,次の条
件でガスクロマトグラフィー〈2.02〉により試験を行う.試
料溶液のクロロホルムのピーク面積は,標準溶液(1)のクロ
ロホルムのピーク面積より大きくない.また,試料溶液及び
標準溶液(2)の内標準物質のピーク面積に対する酢酸エチル
のピーク面積の比Q T及びQ Sを求める.次式により酢酸エチ
ルの量を求めるとき,6.0%以下である.
酢酸エチル(C4H8O2)の量(%)= MS/MT × Q T/Q S × 2
MS:酢酸エチルの秤取量(g)
MT:本品の秤取量(g)
内標準溶液 1-プロパノールのN,N-ジメチルホルムア
ミド溶液(3→200)
説明
Colchicine is a pale-yellow powder that is obtained from various species of Colchicum, primarily Colchicum
autumnale L. Its total chemical synthesis has been achieved, but the primary source of colchicine currently remains alcohol
extraction of the alkaloid from the corm and seed of C. autumnale L. It darkens on exposure to light and possesses
化学的特性
Colchicine is a pale yellow powder. It has little or no odor. It darkens on contact with light.
物理的性質
Appearance: colchicine exists in white or light-yellow crystal powder with no smell, and it is seldom prone to absorb moisture. Melting point: it becomes dark when it is exposed to light, and it melts at 87–89?°C. Solubility: this product is soluble in chloroform or ethanol and it dissolves in water. However, the semihydrate crystal can form in certain concentrations. The product is hardly soluble in ether. Specific optical rotation: ?121° (0.9?g/100?mL, chloroform, 589.3?nm, 17?°C).
来歴
Meadow saffron (Colchicum) is recorded to treat rheumatic swelling on ancient
Egyptian medical papyrus in 1500 B.C.. According to De Materia Medica written by Pedanius Dioscorides in the first century, extract of Meadow saffron is used
in treating gout. London Pharmacopoeia in 1618 recorded that colchicine is also
applied to treat gout.
In 1820, the ingredient was first isolated by the French chemist P.S. Pelletier and
J.B. Caventou. In 1833, it was purified and named by Geiger. Michael Dewar
guessed that there are two seven-membered rings in colchicine in 1945. Murray
Vernon King et al. determined the structure of colchicine by X-ray diffraction in
1952. In 1959, Albert Eschenmoser integrated the product successfully
Colchicine tablet and raw material are approved mostly in domestic in 2010. The
tablet produced by Taiwan manufacturers is approved for being listed in mainland
of China in 2012. The raw material made by Indian obtained the approval in 2013.
There are three kinds of colchicine approved by FDA: with the combination of probenecid, it is prior to be approved. The others are tablet (2009) and capsule (2014).
使用
Colchicine is present in the poisonous autumncrocus (meadow saffron). It is the major alkaloid of Colchicum autumnale L. and Liliaceae. It was used in poison potions in theancient kingdom of Colchis (Greece). It isused therapeutically as an antineoplast, for thesuppression of gout, and in the treatment ofMediterranean fever. It is used in plant studiesfor doubling chromosome groups.
適応症
Colchicine, an alkaloid obtained from the autumn
crocus, has long been used and is relatively selective for
the treatment of acute gouty arthritis. Unlike many of
the newer agents for use in gout, colchicine has minimal
effects on uric acid synthesis and excretion; it decreases
inflammation associated with this disorder. It is thought
that colchicine somehow prevents the release of the
chemotactic factors and/or inflammatory cytokines from
the neutrophils, and this in turn decreases the attraction
of more neutrophils into the affected area .The
ability of colchicine to bind to leukocyte microtubules
in a reversible covalent complex and cause their depolymerization
also may be a factor in decreasing the
attraction of the motile leukocytes into the inflamed
area.
定義
colchicine: An alkaloid derivedfrom the autumn crocus, Colchicumautumnale. It inhibits cell division.Colchicine is used in genetics, cytology,and plant breeding research andalso in cancer therapy to inhibit celldivision.
生物学の機能
Acting on
polymorphonuclear leukocytes and diminishing phagocytosis, it inhibits the production of lactic acid, causing an
increase in the pH of synovial tissue and, thus, a decrease in urate deposition, because uric acid is more soluble at
the higher pH. Additionally, colchicine inhibits the release of lysosomal enzymes during phagocytosis that also
contributes to the reduction of inflammation. Because colchicine does not lower serum urate levels, it has been found
to be beneficial to combine colchicine with a uricosuric agent, particularly probenecid. It is a potent drug, being
effective at doses of approximately 1 mg, but doses as small as 7 mg have caused fatalities.
一般的な説明
Colchicine is an alkaloid isolated from the dried corns andseeds of Colchicum autumnale L., commonly known as autumncrocus or meadow saffron.It is specifically indicated for acute treatment of goutyarthritis because of its ability to block the production and releaseof the CCF that mediates the inflammatory responsebecause of urate crystals, a mechanism different fromcolchicine’s antimitotic action, which is being investigatedfor its anticancer properties. It is often quite effective inaborting an acute gouty attack if given within the first 10 to12 hours after the onset of arthritis.
空気と水の反応
Slowly hydrolyzed in acidic solution, but unbuffered solutions are stable at 68°F for at least six months. Isomerizes on exposure to ultraviolet radiation.
反応プロフィール
Colchicine darkens on exposure to light. Incompatible with strong oxidizing agents. Also incompatible with mineral acids .
危険性
As little as 20 mg may be fatal if ingested.
健康ハザード
Colchicine is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg, i.e. less than 7 drops for a 70 kg (150 lb.) person. Death results from respiratory arrest. The fatal dose varies considerably; as little as 7 mg of Colchicine has proved fatal.
火災危険
Stable.
生物活性
Plant-derived alkaloid that binds to tubulin and depolymerizes microtubules.
作用機序
Colchicine is rapidly absorbed after oral administration
and tends to concentrate in the spleen, kidney,
liver, and gastrointestinal tract. Leukocytes also avidly
accumulate and store colchicine even after a single intravenous
injection. Since colchicine can accumulate in
cells against a concentration gradient, it is postulated
that an active transport process may be involved in its
cellular uptake. The drug is metabolized, primarily in
the liver, by deacetylation. Fecal excretion plays a major
role in colchicine elimination, since it and its metabolites
are readily secreted into the bile. Only about 15 to
30% of the drug is eliminated in the urine except in patients
with liver disease; urinary excretion is more important
in these individuals.
薬理学
The drug
can be given intravenously as well as orally, but care
must be exercised, since extravasated drug may result in
local sloughing of skin and subcutaneous tissues. Relief
of pain and inflammation usually occurs within 48
hours. Small doses of colchicine can be used during
asymptomatic periods to minimize the reappearance or
severity of acute attacks. It should be used with caution
in patients with preexisting compromised heart, kidney,
gastrointestinal tract, and liver disease.
Diarrhea, nausea, vomiting, and abdominal pain are
the major limiting side effects that ultimately determine
the tolerated dosage. These symptoms occur in approximately
80% of patients who take colchicine, especially in those taking high dosages. The hepatobiliary recycling
of colchicine and its antimitotic effect on cells that
are rapidly turning over, such as those of the intestinal
epithelium, account for its gastrointestinal toxicity.
Gastrointestinal symptoms generally intervene before
the appearance of more serious toxicity and thereby
provide a margin of safety in drug administration.
Ingestion of large doses of colchicine may be followed
by a burning sensation in the throat, bloody diarrhea,
shock, hematuria, oliguria, and central nervous system
(CNS) depression.
薬物動態学
Colchicine is absorbed on oral administration, with peak plasma levels being attained within 0.5 to 2 hours after
dosing. Plasma protein binding is only 31%. It concentrates primarily in the intestinal tract, liver, kidney, and spleen
and is excreted primarily in the feces, with only 20% of an oral dose being excreted in the urine. It is retained in the
body for considerable periods of time, being detected in the urine and leukocytes for 9 to 10 days following a single
dose.
抗がん研究
It is a natural toxic secondary metabolite, extracted from Colchicum genus plants. Itprevents gastric cancer by upregulating the dual specificity phosphatase 1 (DUSP1)gene. It is also reported to upregulate transforming growth factor beta 2 (TGF-β2)and A-kinase anchoring protein 12 (AKAP12) in hepatocellular carcinoma (Singhet al. 2016b).
臨床応用
The major use of colchicine is as an antiinflammatory
agent in the treatment of acute gouty arthritis; it is not effective
in reducing inflammation in other disorders. It also
can be used to prevent attacks. Since colchicine is so rapidly
effective in relieving the acute symptoms of gout
(substantial improvement is achieved within hours), it
has been used as a diagnostic aid in this disorder.
Therapy with colchicine is usually begun at the first
sign of an attack and is continued until symptoms subside,
adverse gastrointestinal reactions appear, or a
maximum dose of 6 to 7 mg has been reached.
副作用
Colchicine may produce bone marrow depression, with long-term therapy resulting in thrombocytopenia or aplastic
anemia. At maximum dose levels, GI disturbances (e.g., nausea, diarrhea, and abdominal pain) may occur. Acute
toxicity is characterized by GI distress, including severe diarrhea resulting in excessive fluid loss, respiratory
depression, and kidney damage. Treatment normally involves measures that prevent shock as well as morphine and
atropine to diminish abdominal pain. A number of drug interactions have been reported. In general, the actions of
colchicine are potentiated by alkalinizing substances and are inhibited by acidifying drugs, consistent with its
mechanism of action of increasing the pH of synovial fluid. Responses to CNS depressants and to sympathomimetic
drugs appear to be enhanced. Clinical tests may be affected; most notably, elevated alkaline phosphatase and SGOT
(serum glutamate oxaloacetate transaminase) values and decreased thrombocyte values may be obtained.
安全性プロファイル
experimentally by most routes. Human
systemic effects: aplastic anemia, blood
pressure depression, body temperature
decrease, changes in kidney tubules,
dyspnea, flaccid paralysis without anesthesia,
gastrointestinal effects, kidney damage and
hemorrhaging, muscle contraction or
spasticity, muscle weakness, nausea or
vomiting, respiratory stimulation, and
somnolence. An experimental teratogen.
Experimental reproductive effects. A severe
eye irritant. Human mutation data reported.
Inhibits the formation of microtubules and
thus impairs cell division. When heated to
decomposition it emits toxic fumes of NOx.
職業ばく露
Colchicine is a drug used to treat gouty arthritis, pseudogout, sarcoidal arthritis; and calcific tendonitis.
環境運命予測
Colchicine binds to tubulin and prevents its polymerization
into microtubules, subsequently disrupting microtubule function.
Consequently, it alters nuclear structure, intracellular
transport, and cytoplasmic motility, ultimately causing cell
death. Colchicine is a potent inhibitor of cellular mitosis.
代謝
Metabolism occurs primarily in the liver, with the major metabolite being the amine resulting from amide
hydrolysis.
輸送方法
UN1544 Alkaloids, solid, n.o.s. or Alkaloid salts, solid, n.o.s. poisonous, Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required. UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
純化方法
Commercial material contains up to 4% desmethylcolchicine. Purify colchicine by chromatography on alumina and eluting with CHCl3 [Ashley & Harris J Chem Soc 677 1944]. Alternatively, an acetone solution on alkali-free alumina has been used, and eluting with acetone [Nicholls & Tarbell J Am Chem Soc 75 1104 1953]. It crystallises as yellow needles from EtOAc or CHCl3 with solvent of crystallisation which can be removed at ~70o. It is soluble in Et2O (0.5%), *C6H6 (1%) and H2O (4%). It is sold as “Colgout” for the treatment of gout and binds to tubulin. [Schreiber et al. Helv Chim Acta 44 540 1961, Scott et al. Tetrahedron 21 3605 1965, van Tamelen et al. Tetrahedron 14 8 1961, Beilstein 14 IV 946.]
不和合性
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, mineral acids. Keep away from light.
コルヒチン 上流と下流の製品情報
原材料
準備製品
Acetamide, N,N'-[(7S,7bR,8aS,8bS,9aR,10S,16cS,16dR,16eR,16fS)-5,6,7,7b,8,8a,8b,9,9a,10,11,12,16c,16d,16e,16f-hexadecahydro-1,2,3,8a,8b,14,15,16-octamethoxy-8,9-dioxobisbenzo[3',4']cyclohepta[1',2':3,4]cyclobuta[1,2-c:1',2'-c']cyclobuta[1,2-a:4,3-a']dicyclopentene-7,10-diyl]bis-
γ-ルミコルキシン
(S)-7-アセチルアミノ-6,7-ジヒドロ-10-メチルチオ-1,2,3-トリメトキシベンゾ[a]ヘプタレン-9(5H)-オン