DC(BZ) Β-シアノエチルホスホロアミダイト(異性体混合物) 化学特性,用途語,生産方法
外観
白色〜わずかにうすい黄色, 結晶製粉末〜粉末
溶解性
アセトニトリルによく解ける。
使用上の注意
吸水性が高いので、開封後はできるだけ早めにご使用ください。不活性ガス封入
化学的特性
DMT-dC(bz) Phosphoramidite, also known as 5'-O-DMT-N4-Benzoyl-2'-Deoxycytidine-CE Phosphoramidite or (2R,3R,4R,5R)-2-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-5-(2-isobutyramido-6-oxo-1,6-dihydro-9H-purin-9-yl)-4-(2-methoxyethoxy)tetrahydrofuran-3-yl (2-cyanoethyl) diisopropylphosphoramidite (IUPAC Name), is a novel nucleoside amidite analog, which can be subjected in the synthesis of DNA.
DMT-dC(bz) Phosphoramidite belongs to the group of DNA Phosphoramidites.Its key features include:
Exocyclic amine functions are protected by a benzoyl group (dA(bz) anddC(bz)) or isobutyryl group (dG(ib))
Recommended cleavage and deprotection conditions are 8 hours at 55 °Cor 24 hours at room temperature using concentrated ammonia solution, for standard base-protected oligonucleotides
The high coupling efficiency of Proligo′s DNA phosphoramidites leads to high-yield and high-quality oligonucleotides.
使用
5'-O-DMT-N4-Benzoyl-2'-deoxycytidine 3'-CE phosphoramidite is used to prepare antisense oligonucleotides containing conformationally constrained methoxyaminomethylene and aminooxymethylene and aminomethylene bridged nucleoside analogs.
合成
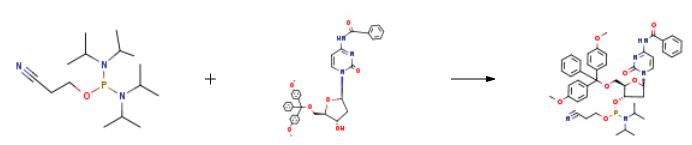
These Examples illustrate the phosphitylation of several protected nucleoside reagents with 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite in the presence of several activators according to the present invention. Eleven phosphitylation reactions (1-11) comprising reacting a protected nucleoside reagent with 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite in the presence of an acid-base activator according to the present invention were conducted, and the product yields of each calculated, as described in the General Procedure, below. The various combinations of protected nucleoside, activator base, activator acid, solvent, and yield for each of the 11 reactions are listed in Table 1. General Procedure: The activator base (1.1 to 1.2 equivalents) is added to the solvent and 0.95 to 1.1 equivalents of activator acid is subsequently added thereto at ambient temperature to form the activator solution. About 1 equivalent of the protected nucleoside is dissolved in about 10 equivalents of the solvent in a separate vessel and about 3 equivalents of the solvent is then distilled off under reduced pressure. About 1 to 1.2 equivalents of 2-Cyanoethyl-N,N,N',N'-tetraisopropylphosphordiamidite is added to the nucleoside mixture at ambient temperature, and the activator solution prepared previously is then added to the nucleoside mixture at ambient temperature with vigorous stirring. After 12 hours, the reaction mixture is diluted with toluene and washed with water. The organic layer is separated, dried over sodium sulfate if necessary, and concentrated under reduced pressure. The yield of the desired amidite is then calculated using HPLC techniques, that is, the resulting product mixture is run through an HPLC column using an appropriate eluent, and the area under the HPLC peaks used to determine the %yield of product in the mixture.
DC(BZ) Β-シアノエチルホスホロアミダイト(異性体混合物) 上流と下流の製品情報
原材料
準備製品