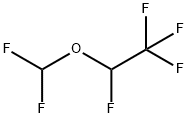
데스플루란
|
|
데스플루란 속성
- 끓는 점
- 23-24°C
- 밀도
- 1,47 g/cm3
- 굴절률
- 1.3577 (estimate)
- 용해도
- 물에 거의 녹지 않으며 무수 에탄올과 섞입니다.
- 물리적 상태
- 액체
- 색상
- 투명한
- CAS 데이터베이스
- 57041-67-5(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 안전지침서 | 23 | ||
| 위험 참고 사항 | Irritant | ||
| 위험 등급 | GAS | ||
| HS 번호 | 2909191800 | ||
| 유해 물질 데이터 | 57041-67-5(Hazardous Substances Data) |
데스플루란 C화학적 특성, 용도, 생산
개요
Desflurane is a new inhalation anesthetic introduced for induction and maintenance of general anesthesia in adults. Due to reports of respiratory irritation, desflurane may be used only for maintenance in children. Although almost structurally identical to isoflurane, a halogen replacement gives desflurane an improved pharmacokinetic profile. It is less soluble in blood and tissue and produces a fast onset of action and a more rapid recovery from anesthesia.화학적 성질
Desflurane is a clear, colourless, mobile, heavy liquid. It is similar to isoflurane (CHF2-O-CHCl-CF3) in that both are halogenated compounds of methyl ethane, except that chlorine is replaced with fluorine in the α-ethyl portion. The halogenation of fluorine reduces the solubility of blood and tissues and alters the boiling point, vapor pressure and stability of desflurane, enhancing its molecular stability as well as its resistance to biodegradation and alkaline degradation.역사
Desflurane was first synthesized by Russel et al U.S. at the 29. of July 1975. The synthesis was started by using floural methyl hemiacetal (CF3CH(OH)OCH3) and converting it to 1,2,2,2- tetraflouroethyl methyl ether (CF3CHFOCH3). Next step was chlorinating two hydrogen atoms of the methyl-group to CF3CHFOCHCl2. This next to last molecule was charged with HF in the presence of antimony pentachloride (SBCL5) to Desflurane. But this way of synthesizing Desfluran was not usable for industrial synthesis, because of the less yield of Desflurane and the expensive educts.용도
Anesthetic.제조 방법
Isoflurane and bromine trifluoride were reacted overnight at room temperature to synthesize desflurane in 62% yield.Biological Functions
Desflurane (Suprane) shares most of the pharmacological properties of isoflurane. Desflurane has low tissue and blood solubility compared with other halogenated hydrocarbons, and its anesthetic partial pressure is thus established more rapidly. Recovery is similarly prompt when the patient is switched to room air or oxygen. Desflurane’s popularity for outpatient procedures stems from its rapid onset and prompt elimination from the body by exhalation. A disadvantage is that desflurane irritates the respiratory tract; thus, it is not preferred for induction of anesthesia using an inhalational technique. However, desflurane may be used to maintain anesthesia after induction with an alternative IV or inhalational agent, preserving the advantage of rapid recovery.Desflurane, like other halogenated hydrocarbon anesthetics, causes a decrease in blood pressure.The reduced pressure occurs primarily as a consequence of decreased vascular resistance, and since cardiac output is well maintained, tissue perfusion is preserved.
Desflurane stimulates the sympathetic nervous system and causes abrupt transient tachycardia during induction or as the concentration of the agent is raised to meet the patient’s changing needs.
Desflurane causes an increase in the rate of ventilation, a decrease in tidal volume, and a decrease in minute volume as inspired concentrations only slightly exceed 1 MAC. Thus should anesthesiologists require desflurane to be administered near or above MAC levels, patients are likely to have marked reductions in PCO2.
일반 설명
Desflurane is a nonflammable, colorless, very volatile liquidpackaged in amber-colored vials. The boiling point is22.8°C, and it requires a vaporizer specifically designed fordesflurane. The manufacturer states that the vials can bestored at room temperature. Desflurane has a blood:gas partitioncoefficient of 0.42, an MAC of 7.3% and an oil:gaspartition coefficient of 18.7. The low blood:gas partition coefficientleads to fast induction times and short recoverytimes. Desflurane is not recommended for induction anesthesiain children because of the high incidence of laryngospasms(50%), coughing (72%), breath holding (68%),and increase in secretions (21%). Desflurane can producea dose-dependent decrease in blood pressure and concentrationsexceeding 1 MAC may cause transient increases inheart rate. Desflurane can react with desiccated carbon dioxideabsorbents to produce carbon monoxide that may resultin elevated levels of carboxyhemoglobin.24.신진 대사
Desflurane is not metabolized to any great extent and, therefore, has not been associated with hepatotoxicity or nephrotoxicity. Metabolites, mostly trifluoroacetate, account for less than 0.02% of the administered dose. Whereas desflurane can react with soda lime or Baralyme to form carbon monoxide, no reports of adverse outcomes in patients have appeared.데스플루란 준비 용품 및 원자재
원자재
준비 용품
데스플루란 공급 업체
글로벌( 53)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-18612256290 |
1056@dideu.com | China | 3131 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; |
laboratory@coreychem.com | China | 30255 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 |
1026@dideu.com | China | 28251 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 18958018566; |
info@afinechem.com | China | 15377 | 58 |
| Alfa Chemistry | +1-5166625404 |
Info@alfa-chemistry.com | United States | 21317 | 58 |
| Amadis Chemical Company Limited | 571-89925085 |
sales@amadischem.com | China | 131981 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 |
jkinfo@jkchemical.com | China | 96815 | 76 |
| LGM Pharma | 1-(800)-881-8210 |
inquiries@lgmpharma.com | United States | 2127 | 70 |
데스플루란 관련 검색:
1,1,1,2-테트라플루오로에테인 헥사플루오로프로펜 산화물
2H-PERFLUORO-5-METHYL-3,6-DIOXANONANE
PERFLUORO-2,5,8,11-TETRAMETHYL-3,6,9,12-TETRAOXAPENTADECANOYL FLUORIDE
2H-PERFLUORO-5,8,11-TRIMETHYL-3,6,9,12-TETRAOXAPENTADECANE
METHYL PERFLUORO(2-METHYL-3-OXAHEXANOATE)
PERFLUORO-2,5,8-TRIMETHYL-3,6,9-TRIOXADODECANOYL FLUORIDE
PERFLUORO(2,5-DIMETHYL-3,6-DIOXANONANOIC) ACID METHYL ESTER
2H-PERFLUORO-5,8-DIMETHYL-3,6,9-TRIOXADODECANE
1-IODO-1-(TRIFLUOROMETHOXY)TETRAFLUOROETHANE
PERFLUORO-2,5,8-TRIMETHYL-3,6,9-TRIOXADODECANOIC ACID METHYL ESTER
2H-PERFLUORO-5,8,11,14-TETRAMETHYL-3,6,9,12,15-PENTAOXAOCTADECANE
2-(HEPTAFLUOROPROPOXY)TETRAFLUOROPROPIONYL FLUORIDE
HFPO PENTAMER, METHYL ESTER
1H,1H-PERFLUORO-2,5,8-TRIMETHYL-3,6,9-TRIOXADODECAN-1-OL
2,5-BIS(TRIFLUOROMETHYL)-3,6-DIOXAUNDECAFLUORONONANOYL FLUORIDE
HEPTAFLUOROPROPYL 1,2,2,2-TETRAFLUOROETHYL ETHER
PERFLUORO-2,5,8,11,14-PENTAMETHYL-3,6,9,12,15-PENTAOXAOCTADECANOYL FLUORIDE