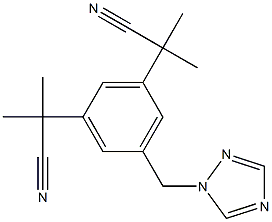
아나스트로졸
|
|
아나스트로졸 속성
- 녹는점
- 81-82°C
- 끓는 점
- 469.7±55.0 °C(Predicted)
- 밀도
- 1.08±0.1 g/cm3(Predicted)
- 저장 조건
- room temp
- 용해도
- DMSO: 용해성40mg/mL
- 산도 계수 (pKa)
- 2.62±0.10(Predicted)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- BCS Class
- 1 (LogP), 3 (CLogP)
- InChI
- InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
- InChIKey
- YBBLVLTVTVSKRW-UHFFFAOYSA-N
- SMILES
- C(#N)C(C1=CC(CN2C=NC=N2)=CC(C(C#N)(C)C)=C1)(C)C
- CAS 데이터베이스
- 120511-73-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-22-61-60 | ||
| 안전지침서 | 26-37/39 | ||
| 유엔번호(UN No.) | 3249 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | CZ1465000 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 29339980 | ||
| 유해 물질 데이터 | 120511-73-1(Hazardous Substances Data) |
아나스트로졸 C화학적 특성, 용도, 생산
화학적 성질
Crystalline Solid. soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of anastrozole in these solvents is approximately 20, 13, and 14 mg/ml.용도
Anastrozole is a nonsteroidal inhibitor of aromatase which effectively blocks estrogen synthesis in postmenopausal women and is used as therapy of estrogen receptor positive breast cancer. Anastrozole has been associated with a low rate of serum enzyme elevations during therapy and rare instances of clinically apparent liver injury.정의
ChEBI: Anastrozole is a 1,2,4-triazole compound having a 3,5-bis(2-cyano-2-propyl)benzyl group at the 1-position. It has a role as an antineoplastic agent and an EC 1.14.14.14 (aromatase) inhibitor. It is a member of triazoles and a nitrile.제조 방법
synthesis of anastrozole can be realized in four steps based on 3,5-bis(bromomethyl)toluene. Starting with a S N 2 displacement using potassium nitrile and tetrabutylammonium bromide as a phase transfer catalyst to give bis-nitrile compound. Bis-nitrile compound formed undergoes deprotonation with NaH and methylated afterwards with methyl iodide to give bis-dimethyated product.Product undergoes radical substitution reaction following the Wohl-Ziegler reaction using N-bromosuccinamide and benzoyl peroxide as the radical initiator.In the final step, benzylbromide undergoes SN2 displacement with sodium triazole to give anastrozole.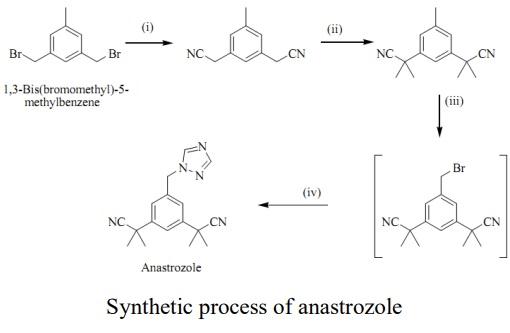
주요 응용
Anastrozole (aromatase inhibitor) has been used:as a positive control in DNA fragmentation (ladder) assay
to investigate its effects along with extra virgin olive oil and its major fatty acid component (omega-9 OA) in estrogen receptor positive mammary adenocarcinoma cells
to study its effects on viability, cell proliferation and apoptosis in Glioblastoma multiforme model in vivo
일반 설명
Anastrozole(120511-73-1) is a non-steroidal and expensive drug marketed under the trade name Arimidex. It was the first specific aromatase inhibitor approved in theUnited States. It is indicated for first-line treatment of postmenopausalwomen with advanced or metastatic breast cancer,for second-line treatment of postmenopausal patientswith advanced breast cancer who have had disease progressionfollowing tamoxifen therapy, and for adjuvant treatmentof women with early breast cancer. Patients who did not respondto tamoxifen therapy rarely respond to anastrozole.생물학적 활성
Potent and highly selective aromatase (CYP19) inhibitor (IC 50 = 15nM) that has no discernible effect on adrenocorticoid hormone synthesis. Reduces plasma estrogen levels and exhibits antitumor activity in vivo . Orally active.Mechanism of action
Anastrozole, a benzyltriazole derivative, competes with the natural s ubstrate for binding to the active site of the aromatase. The mechanism of enzyme inhibition resides in the coordination of the triazole ring with the hemeiron atom of the aromatase enzyme complex. This coordination ultimately prevents arom atization of androgens into estrogens and, therefore, deprives the tumor of estrogen. This effect is reversible. In the presence of anastrozole, estradiol levels are reduced to undetectable levels, with no adverse effects on levels of any other horm one, including cortisol and aldosterone. Maximal estrogen suppression is produced by a 1mg dose. Estrogen suppression is maintained for up to 6 days after discontinuing anastrozole.Pharmacokinetics
Anastrozole is well absorbed orally, with biliary elimination as its primary route (85%) and an elimination half-life of approxim ately 50 hours. Approximately 60% of an oral dose is m etabolized in the liver by N-dealkylation, hydroxylation, and glucuronidation to inactive triazole metabolites.Clinical Use
Anastrozole is a potent and highly selective, nonsteroidal aromatase inhibitor utilized in the treatment of advanced breast cancer that is horm one-responsive. It is considered to be second-line therapy (after tamoxifen) in the treatment of postm enopaus al breast cancer.부작용
The most common anastrozole side effects are related to lower estrogen levels in the body. They include hot flashes, nausea and vomiting, and mood changes. Anastrozole could cause your bones to thin, which raises your risk of osteoporosis. It can also cause high cholesterol.환경귀착
Anastrozole is classified as readily biodegradable and is moderately mobile in soils. The measured octanol-water partition coefficient is low, therefore anastrozole is not predicted to bioaccumulate in aquatic organisms.참고 문헌
[1] DUKESM. The preclinical pharmacology of “Arimidex” (anastrozole; ZD1033)–a potent, selective aromatase inhibitor.[J]. Journal of Steroid Biochemistry and Molecular Biology, 1996. DOI:10.1016/0960-0760(96)00064-7.[2] U B. Anastrozole: a new addition to the armamentarium against advanced breast cancer.[J]. American Journal of Clinical Oncology-Cancer Clinical Trials, 1998. DOI:10.1097/00000421-199804000-00014.
[3] L?NNINGP E DowsettM GeislerJ. Pharmacological and clinical profile of anastrozole.[J]. Breast Cancer Research and Treatment, 1998. DOI:10.1023/a:1006000806630.
[4] HOZUMIYASUO. Effects of anastrozole on lipid metabolism compared with tamoxifen in rats.[J]. Breast Cancer Research and Treatment, 2002. DOI:10.1023/a:1020571617274.
아나스트로졸 준비 용품 및 원자재
원자재
5-Methyl-1,3-benzenediacetonitrile
수소화나트륨
N-브로모숙신산이미드
1,2,4-트리아졸
N,N-다이메틸폼아마이드
사염화탄소
나트륨 트리아졸
초산에틸
석유 에테르
요오드 메탄
사이안화 칼륨
과산화 벤조일
3,5-Bis(bromomethyl)toluene
준비 용품
아나스트로졸 공급 업체
글로벌( 676)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Wuhan Fortuna Chemical Co., Ltd | +86-27-59207850 +86-13986145403 |
info@fortunachem.com | China | 5988 | 58 |
| Shenzhen Monkono Technology Co.,Ltd | +86-17063441314 |
sansbiotech@outlook.com | China | 677 | 58 |
| Wuhan Cell Pharmaceutical Co., Ltd | +86-13129979210 +86-13129979210 |
sales@cellwh.com | China | 376 | 58 |
| Doublewin Biological Technology Co., Ltd | +86-19971437628 |
doublewin-bella@nandrolonesteroid.com | China | 97 | 58 |
| qingdao future trading Co., Ltd | +86-13335044410 +86-13335044410 |
cui56813@163.com | China | 110 | 58 |
| Guangdong Tuoyuan Biotechnology Co., LTD | +85267545345 |
sterodschina@gmail.com | China | 129 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5993 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 |
admin@hbdangtong.com | China | 991 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 |
admin@firsky-cn.com | China | 436 | 58 |
아나스트로졸 관련 검색:
디페닐아세토니트릴 메틸-1H-벤조트리아졸 페닐아세토나이트릴
p-Nitrophenylacetonitrile
4-BROMO-2,2-DIPHENYLBUTYRONITRILE
5-Methyl-1H-benzotriazole
2,2-(5-methyl-1,3-phenylene)di(2-methylpropiononitrile (intermediate of anastrozole)
3,5-Dimethylphenylacetonitrile
3,5-Bis(2-cyanoprop-2-yl)benzyl bromide
1,3-Phenylenediacetonitrile
3-Methylbenzyl cyanide
Anastrozole IMpurity (3-(1-Cyano-1-Methylethyl)-alfa,alfa-diMethyl-5-(1H-,1,2,4-triazole-1-ylMethyl)-benzeneacetic acid)
2-[3-(2-cyanopropan-2-yl)-5-(1,2,4-triazol-4-ylmethyl)phenyl]-2-methyl-propaneni
5-Methyl-1,3-benzenediacetonitrile
Anastrozole N-glucuronide
Anastrozole iMpurity
2-[3-CHLOROMETHYL-5-(CYANO-DIMETHYL-METHYL)-PHENYL]-2-METHYL-PROPIONITRILE
3,5-DI-TERT-BUTYL-BENZYLAMINE