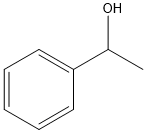
알파-메틸벤질 알코올 C화학적 특성, 용도, 생산
개요
α-Methylbenzyl alcohol has a mild hyacinth-gardenia odor.
화학적 성질
DL-1-Phenethylalcohol has been
identified as a volatile component of food (e.g., in tea aroma and mushrooms).
The alcohol is a colorless liquid with a dry, rose-like odor, slightly reminiscent
of hawthorn. It can be prepared by catalytic hydrogenation of acetophenone. 1-
Phenylethyl alcohol is used in small quantities in perfumery and in larger amounts
for the production of its esters, which are more important as fragrance materials.
출처
Two optically active isomers exist; the commercial product is the racemic form. Reported found in cranberry,
grapes, chive, Scotch spearmint oil, cheeses, cognac, rum, white wine, cocoa, black tea, filbert, cloudberry, beans, mushroom and
endive.
용도
The Arthrobacter sp. is able to grow with (+ I-,(-)-1-phenylethanol or the racemic mixture as sole source of carbon. Growth is most rapid with the (-)-isomer, doubling time 12 h. Lipases show good activity and, in some cases, improved enantioselectivity when employed in pure ionic liquids for dynamic kinetic resolution of 1-phenylethanol by transesterification.
생산 방법
1-Phenylethanol is coproduced with propylene oxide by
reaction of a-peroxyethylbenzene (formed by the oxidation
of ethylbenzene) with propylene. It is used as a fragrance additive in cosmetics such as perfumes, creams,
and soaps and is an intermediate in styrene production.
1-Phenylethanol is also added to foods as a flavoring agent. Industrial exposure may occur from dermal contact and ingestion.
제조 방법
By oxidation of ethylbenzene or by reduction of acetophenone.
정의
ChEBI: An aromatic alcohol that is ethanol substituted by a phenyl group at position 1.
일반 설명
A colorless liquid. Insoluble in water and less dense than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
공기와 물의 반응
Insoluble in water.
반응 프로필
Attacks plastics. [Handling Chemicals Safely, 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: An explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M. 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
건강위험
Irritating to the skin, eyes, nose, throat, and upper respiratory tract.
화재위험
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
Poison by ingestion and subcutaneous routes. Moderately toxic by skin contact. A skin and severe eye irritant. Questionable carcinogen. Combustible when exposed to heat or flame; can react with oxidming materials. To fight fire, use alcohol foam, foam, CO2, dry chemical
Carcinogenicity
In an NTP study, both sexes of F344 rats were dosed by gavage with 0, 375, and 750 mg/kg
1-phenylethanol 5 days/week for 2 years. There was an
increased incidence of neoplastic kidney tumors in the
high-dose male rats but no evidence of carcinogenicity in
the female rats . In the same NTP study, both sexes of
B6C3F1 mice were dosed by oral gavage with 0, 375, and
750 mg/kg 1-phenylethanol 5 days/week for 2 years. There
was no evidence that 1-phenylethanol was carcinogenic to
mice in this study.
Purification Methods
Purify the alcohol via its hydrogen phthalate. [See Houssa & Kenyon J Chem Soc 2260 1930.] Shake it with a solution of ferrous sulfate, and th
알파-메틸벤질 알코올 준비 용품 및 원자재
원자재
준비 용품