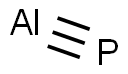인화 알루미늄 C화학적 특성, 용도, 생산
개요
Aluminum phosphide, is a binary salt, one of the NCHP acronyms (see Chapter 2). These salts have the specific hazard of giving off poisonous and pyrophoric phosphine gas when in contact with moist air, water, or steam. They will also ignite spontaneously upon contact with air. This compound is composed of gray or dark yellow crystals and is a dangerous fire risk. Aluminum phosphide decomposes upon contact with water and has a specific gravity of 2.85, which is heavier than water. The four-digit UN identification number is 1397. The NFPA 704 designation is health 4, flammability 4, and reactivity 2. The white section at the bottom of the diamond has a W with a slash through it, indicating water reactivity. Aluminum phosphide is used in insecticides, fumigants, and semiconductor technology.
화학적 성질
Aluminum phosphide is a pyrophoric, dark gray or dark yellow crystalline solid
용도
Source of phosphine; in semiconductor research; as fumigant.
일반 설명
Aluminum phosphide is a dark gray or dry, yellow, crystalline solid. Aluminum phosphide reacts with moisture to give phosphine, a flammable and poisonous gas. Normally, phosphine will spontaneously ignite upon contact with air. If there is an excess of water, the phosphine fire will not normally ignite any surrounding combustible material.
공기와 물의 반응
Decomposed by water or moist air, evolving phosphine, a toxic gas that often ignites [Merck 11th ed. 1989].
반응 프로필
Aluminum phosphide is a reducing agent. Contact with mineral acids causes explosive evolution of toxic phosphine [Wang, C. C. et al., J. Inorg. Nucl. Chem., 1963, 25, p. 327]. Heating produces highly toxic fumes of phosphorus oxides. Can react vigorously upon contact with oxidizing agents. [Sax, 9th ed., p. 119].
위험도
Dangerous fire risk. It evolves phosphine.
건강위험
Acute toxicity occurs primarily by the inhalation route when Aluminum phosphide decomposes into the toxic gas, phosphine. The human median lethal dose for Aluminum phosphide has been reported to be 20 mg/kg. Rated as super toxic: probable oral lethal dose is less than 5 mg/kg or less than 7 drops for a 70 kg (150 lb.) person.
화재위험
Releases toxic fumes on exposure to moist air, water, or acids. Decomposes to produce phosphine gas. Avoid water, dilute mineral acids, dilute or concentrated hydrochloric acid. Stable when dry. Avoid moist air.
농업용
Fumigant, Fungicide, Rodenticide, Insecticide: Used as an insecticidal fumigant for grain, peanuts,
processed food, animal feed, leaf tobacco, cottonseed, and
as space fumigant for flour mills, warehouses and railcars. It is also used in baits for rodent and mole control in
crops. Used as a source of phosphine; in semiconductor
research. Zinc phosphide is often mixed with bait food
such as cornmeal, which can be a danger to pets and children. When phosphides are ingested or exposed to moisture, they release phosphine gas. A U.S. EPA restricted
Use Pesticide (RUP). Metallic phosphides on clothes,
skin, or hair can react with water or moisture to generate
phosphine gas.
상품명
AL-PHOS®; CELPHIDE®; CELPHOS®;
DELICIA®; DETIA®; DETIA-EX-B®; DETIA GAS
EX®; DETIA-GAS-EX-B®; DELICIA GASTOXIN;
FARMOZ®; FUMITOXIN®; PHOSTOXIN®;
PHOSTOXIN-A®; QUICKPHOS®; QUICK TOX®;
RENTOKIL GASTION®
Safety Profile
A human poison by inhalation and ingestion. Dangerous; in contact with water, steam, or alkali it slowly yields PH3, which is spontaneously flammable in air. Explosive reaction on contact with mineral acids produces phosphine. When heated to decomposition it yields toxic PO,. See also ALUMINUM COMPOUNDS, PHOSPHIDES, and PHOSPHINE.
AHGOOO
잠재적 노출
Used as a rodenticide; wood preservative; as a source of phosphine; as an insecticidal fumigant for grain, peanuts, processed food, animal feed, leaf tobacco, cottonseed; and as space fumigant for flour mills, warehouses and railcars. Used in semiconductor research
환경귀착
Phosphine is known to bind to and inhibit cytochrome oxidase
and changes the valence of the hem component of hemoglobin.
Oxidative stress is one of the main mechanisms of
action of AlP toxicity, which boosts extramitochondrial release
of free oxygen radicals resulting in lipid peroxidation and
protein denaturation of the cell membrane in various organs.
Furthermore, AlP reduces glutathione, which is one of the main
antioxidant defenses. AlP causes toxic stress, accompanied by
changes in glucose metabolism. It also disrupts protein
synthesis and enzymatic activity, particularly in the lung and
heart cell mitochondria, which leads to blockage of the mitochondrial
electron transport chain. Phosphine may cause
denaturing of various enzymes; it is involved in cellular respiration
and metabolism, and may be responsible for denaturation
of the oxyhemoglobin molecule.
운송 방법
UN1397 Aluminum phosphide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material, 6.1-Poisonous materials.
비 호환성
Able to ignite spontaneously in moist air; forms toxic and explosive phosphine gas on contact with moisture in air. Reacts violently with water, steam, carbon dioxide; acids, alcohols, and foam fire extinguishers. Contact with water and bases slowly releases highly flammable and toxic phosphine gas.
폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Allow to react slowly with moisture in the open, being sure that phosphine gas evolved is dissipated. Alternatively, mix with dry diluent and incinerate at temperature above 1000 C with effluent gas scrubbing. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed of properly by following package label directions or bycontacting your local or federal environmental control agency, or by contacting your regional EPA office.
인화 알루미늄 준비 용품 및 원자재
원자재
준비 용품