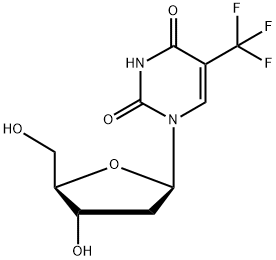
Trifluridine
|
|
Trifluridine 속성
- 녹는점
- 190-193 °C (lit.)
- 밀도
- 1.4365 (estimate)
- 굴절률
- 50 ° (C=1, H2O)
- 저장 조건
- 2-8°C
- 용해도
- DMSO(최대 25mg/ml) 또는 물(최대 14mg/ml)에 용해됩니다.
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- pKa 7.85 (Uncertain)
- 색상
- 회백색
- Merck
- 14,9687
- 안정성
- 제공된 대로 구매일로부터 1년 동안 안정적입니다. DMSO 또는 증류수에 용해된 용액은 -20°C에서 최대 1개월 동안 보관할 수 있습니다.
- CAS 데이터베이스
- 70-00-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22-40 | ||
| 안전지침서 | 22-36 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | XP2087500 | ||
| 위험 참고 사항 | Keep Cold | ||
| HS 번호 | 29349990 | ||
| 유해 물질 데이터 | 70-00-8(Hazardous Substances Data) | ||
| 독성 | LD50 intraperitoneal in mouse: 1931mg/kg |
Trifluridine C화학적 특성, 용도, 생산
개요
Trifluridine (trifluorothymidine, TFT), a fluorinated pyrimidine nucleoside, is an anti-herpesvirus agent and an antitumor antimetabolite agent. It is an analog of thymidine which inhibits thymidylate synthase possesses antiviral and anticancer activity. After phosphorylation by thymidine kinase, it is incorporated into DNA where it induces DNA-damage and interferes with repair enzymes. Enhances frame shift insertion and deletion in CRISPR genome editing in pluripotent stem cells.화학적 성질
White to Off-White Solid용도
Trifluridine is used as anti-herpesvirus antiviral agent in ophthalmie preparations.정의
ChEBI: Trifluridine is a pyrimidine 2'-deoxyribonucleoside compound having 5-trifluoromethyluracil as the nucleobase. An antiviral drug used mainly in the treatment of primary keratoconjunctivitis and recurrent epithelial keratitis. It has a role as an antiviral drug, an antimetabolite, an EC 2.1.1.45 (thymidylate synthase) inhibitor and an antineoplastic agent. It is a nucleoside analogue, an organofluorine compound and a pyrimidine 2'-deoxyribonucleoside.Indications
Trifluridine (Viroptic) is a fluorinated pyrimidine nucleoside that has in vitro activity against HSV-1 and HSV- 2, vaccinia, and to a lesser extent, some adenoviruses. Activation of trifluridine requires its conversion to the 5 monophosphate form by cellular enzymes.Trifluridine monophosphate inhibits the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) by thymidylate synthetase. In addition, it competes with deoxythymidine triphosphate (dTTP) for incorporation by both viral and cellular DNA polymerases. Trifluridine-resistant mutants have been found to have alterations in thymidylate synthetase specificity.일반 설명
Trifluridine, 5-trifluoromethyl-29-deoxyuridine (Viroptic),is a fluorinated pyrimidine nucleoside that demonstrates invitro inhibitory activity against HSV-1 and HSV-2, CMV,vaccinia, and some adenoviruses.Trifluridine possesses atrifluoromethyl group instead of an iodine atom at the 5-position of the pyrimidine ring.synthetase, and the biologically generated triphosphatecompetitively inhibits thymidine triphosphate incorporationinto DNA by DNA polymerase. In addition, trifluridine inits triphosphate form is incorporated into viral and cellularDNA, creating fragile, poorly functioning DNA.Trifluridine is approved in the United States for the treatmentof primary keratoconjunctivitis and recurrent epithelialkeratitis caused by HSV types 1 and 2. Topical trifluridineshows some efficacy in patients with acyclovir-resistantHSV cutaneous infections.
Mechanism of action
Trifluorothymidine is a fluorinated pyridine nucleoside structurally related to idoxuridine. It has been approved by the U.S. FDA and is a potent, specific inhibitor of replication of HSV-1 in vitro. Its mechanism of action is similar to that of idoxuridine. Like other antiherpes drugs, it is first phos-phorylated by thymidine kinase to mono-, di-, and triphosphate forms, which are then incorporated into viral DNA in place of thymidine to stop the formation of late virus mRNA and subsequent synthesis of the virion proteins.Pharmacokinetics
Trifluorothymidine is a synthetic halogenated pyrimidine nucleoside, first synthesized as an antitumor agent. It inhibits enzymes of the DNA pathway and is incorporated into both cellular and progeny viral DNA, causing faulty transcription of late messenger RNA and the production of incompetent virion protein. It does not require a viral thymidine kinase for monophosphorylation and is far less selective and more toxic than other analogs. It is active against HSV-1 and HSV-2, vaccinia virus, CMV and possibly adenovirus. Trifluorothymidine, when given IV, shows a plasma half-life of 18 minutes and is excreted in the urine either unchanged or as the inactive metabolite 5-carboxyuracil. When applied as a 1% ophthalmic solution, it rapidly enters the aqueous humor of HSV-infected rabbits’ eyes but is cleared within 60–90 min.부작용
The most frequent adverse reactions to trifluridine administration are transient burning or stinging and palpebral edema. Other adverse reactions include superficial punctate keratopathy, epithelial keratopathy, hypersensitivity, stromal edema, irritation, keratitis sicca, hyperemia, and increased intraocular pressure.Trifluridine is mutagenic in vitro and carcinogenic and teratogenic when administered subcutaneously to animals. Topical trifluridine was not teratogenic in animal studies. Because it is applied topically in humans, the likelihood of systemic effects is low.
Trifluridine 준비 용품 및 원자재
원자재
준비 용품
Trifluridine 공급 업체
글로벌( 381)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Beijing Ribio Biotech Co.,Ltd | 010-62664360 +8613328773880 |
wucy@ribio.com.cn | China | 117 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 |
2691956269@qq.com | China | 359 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Lianyungang happen teng technology co., LTD | 15950718863 |
wang666xt@163.com | CHINA | 295 | 58 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Biochempartner | 0086-13720134139 |
candy@biochempartner.com | CHINA | 967 | 58 |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 |
admin@nexconn.com | China | 10248 | 58 |