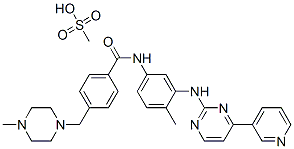
Imatinib mesylate
|
|
Imatinib mesylate 속성
- 녹는점
- 214-224°C
- 저장 조건
- 2-8°C
- 용해도
- H2O: 가용성10mg/mL, 투명
- 물리적 상태
- 흰색 고체
- 색상
- 흰색에서 베이지색
- 안정성
- 제공된 대로 구매일로부터 1년 동안 안정적입니다. DMSO 또는 물에 용해된 용액은 -20°C에서 최대 3개월 동안 보관할 수 있습니다.
- InChIKey
- YLMAHDNUQAMNNX-UHFFFAOYSA-N
- SMILES
- C(NC1=CC=C(C)C(NC2=NC=CC(C3=CC=CN=C3)=N2)=C1)(=O)C1=CC=C(CN2CCN(C)CC2)C=C1.CS(O)(=O)=O
- CAS 데이터베이스
- 220127-57-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | CV5520550 | ||
| HS 번호 | 29350090 | ||
| 유해 물질 데이터 | 220127-57-1(Hazardous Substances Data) |
Imatinib mesylate C화학적 특성, 용도, 생산
개요
In May 2001, the FDA approved imatinib as a new cancer drug after a record review time of just 2.5 months. Imatinib was launched as Gleevec in the US for chronic myelogenous leukemia (CML) in blast crisis, accelerated phase or chronic phase after interferon-alpha failure. This compound can be prepared by a four step sequence from a condensation of the 1-(3-pyridyl)ethanone with dimethyl formamide dimethylacetal, followed by successive cyclization with the methyl-nitrophenyl guanidine, hydrogenolysis and condensation with the benzoyl chloride of the methylpiperazine. Imatinib is the first of a new class of anticancer drugs that are specifically designed to target the molecular pathways involved (oncogenic event) in the development of disease. The Brc-Abl oncoprotein is a constitutively active tyrosine kinase that causes CML. Imatinib is a competitive inhibitor of this tyrosine kinase as well as Abl, Kit and the PDGFR kinases It binds to the ATP-binding site of the target kinase and prevents the transfer of phosphate from ATP to the tyrosine residues of various substrates and consequently blocks the proliferation of the leukemic cells. Phase II studies demonstrated that in chronic phase CML, over 90% of the patients had their leukocyte counts return to normal and 56% had a major cytogenic response. No phase III data is currently available. It is clear from the evidence available that imatinib has advantages over IFN-alpha, such as reduced toxicity, more rapid hematological response, higher rate of cytogenic response and oral administration. The drug is well tolerated, producing few side effects, classified as grade 1 nausea, muscle cramps, diarrhea, edema and vomiting. Imatinib is metabolized primarily by the CYP3A4 enzyme system and drugs capable of modulating this system would be expected to modify the patient's exposure. Novartis expects to launch imatinib for the treatment of gastrointestinal stromal tumors in 2002.화학적 성질
Off-White Solid용도
Imatinib Mesylate is orally bioavailability mesylate salt of Imatinib, which is a multi-target inhibitor of v-Abl, c-Kit and PDGFR with IC50 of 0.6 μM, 0.1 μM and 0.1 μM, respectively. Imatinib also known as Gleevec, Glivec, CGP-57148B, STI-571 & Imatinib정의
ChEBI: A methanesulfonate (mesylate) salt that is the monomesylate salt of imatinib. Used for treatment of chronic myelogenous leukemia and gastrointestinal stromal tumours.일반 설명
Imatinib is available in 100- and 400-mg capsules for oraladministration and is indicated for the treatment of CML,gastrointestinal stromal tumors (GIST) that express Kit andacute lymphoplastic leukemia that is positive for thePhiladelphia chromosome.Bioavailability of the agent is nearly 100% by the oralroute. The agent is highly protein bound and metabolized tothe N-desmethyl derivative by CYP3A4-mediated removalof the piperazinyl methyl group. The resulting metabolite issimilar to the parent in activity. Elimination occurs primarilyin the feces, and the terminal half-life is 18 hours forthe parent and 40 hours of the N-desmethyl metabolite.Resistant forms of the TK are known, which have alteredamino acids that prevent binding. In addition, there may beincreased levels of the kinase itself. The drug is also a substratefor Pgp and an additional efflux transporter known asbreast cancer resistance protein (BCRP), both of which removethe drug from the cell. These transporters are also inhibitedby the agent as well. Severe side effects include ascites,neutropenia, thrombocytopenia, skin rash, andpulmonary edema. Less serious side effects include nausea/vomiting, heartburn, and headache but overall, the agentis better tolerated than most other medications used in treatingthe disease.
Imatinib mesylate 준비 용품 및 원자재
원자재
준비 용품
Imatinib mesylate 공급 업체
글로벌( 755)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 992 | 58 |
| shandong perfect biotechnology co.ltd | +86-53169958659; +8618596095638 |
sales@sdperfect.com | China | 294 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Nanjing Fred Technology Co., Ltd | +86-25-84696168 +86-15380713688 |
Austin@fredbio.com | China | 2449 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7933 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 900 | 58 |
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 |
sales@frappschem.com | China | 885 | 50 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 |
sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
Imatinib mesylate 관련 검색:
2-아미노피리딘 아크릴산메틸 티오판산-메틸 3-아미노피리딘 아세트산 메틸 4-다이메틸아미노피리딘 메틸파라벤 벤설프론 메틸 쿠레톡심메틸 브롬화 메틸 메틸 파라싸이온(메틸 파라티온)
Gatifloxacin mesylate
Pazufloxacin mesilate
Pefloxacin mesylate
Methyl
N-DESMETHYL IMATINIB
4-Chloromethyl-N-[4-methyl-3-[[4-(pyridin-3-yl)pyrimidin-2-yl]amino]phenyl]benzamide
Gemcitabine hydrochloride