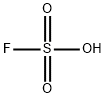
플루오로설폰산
|
|
플루오로설폰산 속성
- 녹는점
- −87.3 °C(lit.)
- 끓는 점
- 165.5 °C(lit.)
- 밀도
- 1.726 g/mL at 25 °C(lit.)
- 증기 밀도
- 3.5 (vs air)
- 증기압
- 2.5 mm Hg ( 25 °C)
- 저장 조건
- 2-8°C
- 용해도
- reacts with H2O
- 산도 계수 (pKa)
- -6.29±0.15(Predicted)
- 물리적 상태
- 무색의 액체
- 색상
- 무색의
- 수용성
- H2O에서 격렬하게 가수분해됩니다. 아세톤에서 붉은빛을 띤 갈색을 띤다 [MER06]
- Merck
- 13,4206
- 안정성
- 안정적인. 유리를 부식시킬 수 있습니다. 많은 금속, 베이스와 호환되지 않습니다.
- CAS 데이터베이스
- 7789-21-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20-35 | ||
| 안전지침서 | 26-45 | ||
| 유엔번호(UN No.) | UN 1777 8/PG 1 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | LP0715000 | ||
| 위험 참고 사항 | Corrosive/Toxic | ||
| 위험 등급 | 8 | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 7789-21-1(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-17058 |
| 그림문자(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | |||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||
| 예방조치문구: |
|
플루오로설폰산 C화학적 특성, 용도, 생산
개요
Fluorosulphuric acid (FSO3H) is a free-flowing colourless fuming liquid. It is soluble in polar organic solvents such as acetic acid, ethyl acetate, and nitrobenzene but poorly soluble in nonpolar solvents such as alkanes. FSO3H is corrosive to metals and body tissues, corrodes glass, and is incompatible with many metals and bases. With its strong acidity, FSO3H dissolves almost all organic compounds that are even weak proton acceptors. FSO3H hydrolyses slowly to HF and sulphuric acid (H2SO4). The related triflic acid (CF3SO3H) retains the high acidity of FSO3H but is more hydrolytically stable. FSO3H is one of the strongest known simple Bronsted acids. FSO3H is useful for regenerating mixtures of HF and H2SO4 for etching lead glass. FSO3H isomerises alkanes and the alkylation of hydrocarbons with alkenes, although it is unclear if such applications are of commercial importance. FSO3H is used as a catalyst in organic synthesis and in electroplating and as a fluorinating agent and as a laboratory fluorinating agent. FSO3H has been reported as a highly toxic and corrosive chemical substance. FSO3H is non-combustible but enhances combustion of other substances. It gives off irritating or toxic fumes (or gases) in a fire. FSO3H hydrolyses to release HF. Addition of water to FSO3H causes very violent reactions similar to the addition of water to H2SO4. Addition of FSO3H to water is a much more violent process than addition of H2SO4. The combination of FSO3H and others is categorised as ‘magic acids and super acids’. Very short contact and the fumes of FSO3H cause severe painful burns.화학적 성질
colourless liquid용도
Catalyst in organic synthesis, electropolishing, fluorinating agent.일반 설명
A fuming liquid. Boiling point 163°C. Density 1.73 g / cm3. Corrosive to metals and to tissue. Both very short contact and the fumes can cause severe painful burns. Used as a catalyst in organic synthesis, in electroplating and as a fluorinating agent.공기와 물의 반응
Fumes in air. Reacts violently with water to form hydrogen fluoride and sulfuric acid mist.반응 프로필
FLUOROSULFONIC ACID is very strongly acidic [Merck]. Reacts exothermically with chemical bases (examples: amines, amides, and inorganic hydroxides). These reactions can generate dangerously large amounts of heat in small spaces. Reacts violently with water to generate hydrofluoric acid and sulfuric acid. Reacts with active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. Generates flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. May catalyze (increase the rate of) chemical reactions.위험도
Extremely irritating to eyes and tissue.건강위험
Inhalation of fumes causes severe irritation of nose and throat. Contact of liquid with eyes or skin causes very severe burns of mouth and stomach.Safety Profile
Probably a poison by inhalation. A corrosive irritant to the skin, eyes, and mucous membranes. See also FLUORIDES, SULFURIC ACID, and FLUOROSULFONATES.플루오로설폰산 준비 용품 및 원자재
원자재
준비 용품
플루오로설폰산 공급 업체
글로벌( 55)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21687 | 55 |
| career henan chemical co | +86-0371-86658258 15093356674; |
sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34571 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 |
eric@witopchemical.com | China | 23556 | 58 |
| Hefei Hirisun Pharmatech Co., Ltd | +8615056975894 |
shawn@hirisunpharm.com | CHINA | 9923 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | CHINA | 52861 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 9020 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
플루오로설폰산 관련 검색:
메틸 플루오로설페이트 트리메틸시릴 트리플루오로메탄설폰산염 플루오로설폰산 수소 펜타플루오로(플루오르황산염)안티몬산염(1-)
TATM
POTASSIUM FLUOROSULFATE
Aluminum trifluoromethanesulfonate
PERFLUOROSULFONIC ACID-PTFE COPOLYMER
AMMONIUM FLUOROSULFATE
10-METHYL-9-(PHENOXYCARBONYL)ACRIDINIUM FLUOROSULFONATE
PENTAFLUOROALLYL FLUOROSULFATE
1,1,1,3,3-PENTAFLUOROISOPROPYL FLUOROSULFATE
DISULFURYL FLUORIDEDISCONTINUED WHEN STOCK SOLD 4/10/00
TETRABUTYLAMMONIUM FLUOROSULFATE
RARECHEM AQ N5 4122
RARECHEM AQ TC 1031
RARECHEM AQ TC 1015
RARECHEM AQ NN 0429