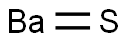황화 바륨
|
|
황화 바륨 속성
- 녹는점
- 1200°C
- 밀도
- 4.25 g/mL at 15 °C(lit.)
- 물리적 상태
- 가루
- Specific Gravity
- 4.25
- 색상
- 옅은 회색에서 노란색까지
- 수용성
- 물에는 용해되지만 알코올에는 용해되지 않습니다.
- 감도
- Moisture Sensitive
- Merck
- 14,995
- 안정성
- 안정적이지만 가열하면 분해됩니다. 산, 산화제, 물과 호환되지 않습니다.
- CAS 데이터베이스
- 21109-95-5(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,N,Xi,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/22-31-50-36/37/38-41-35 | ||
| 안전지침서 | 28-61 | ||
| 유엔번호(UN No.) | UN 2813 4.3/PG 2 | ||
| WGK 독일 | 2 | ||
| TSCA | Yes | ||
| 위험 등급 | 4.3 | ||
| 포장분류 | III | ||
| HS 번호 | 2830908500 | ||
| 기존화학 물질 | KE-02093 |
| 그림문자(GHS): |
  
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||||||||||||||||
| 예방조치문구: |
|
황화 바륨 C화학적 특성, 용도, 생산
화학적 성질
colourless crystals, or white to grey-brown powder,물리적 성질
Colorless crystalline solid; density 4.25 g/cm3; refractive index 2.155; melts at 1,200°C; soluble in water (decomposes); insoluble in alcohol.출처
Barium sulfide occurs in the form of black ash, which is a gray to black impure product obtained from high temperature carbonaceous reduction of barite. It is the starting material in the manufacture of most barium compounds including barium chloride and barium carbonate. It is used in luminous paints; for dehairing hides; as a flame retardant; and for generating H2S.용도
Barium sulfide has different characters, so it is used in a variety of ways, such as in electronics, paint pigments, dehairing hides, flame retardant, luminous paints, and generating pure hydrogen sulfide. Barium sulfide can deviate X-rays and helps to give clearly image of the soft tissue. Barium sulfide is a precursor to other barium compounds and also used as a short wavelength emitters for electronic displays.제조 방법
Barium sulfide can be prepared by the direct reaction of the elements, calcined in an inert atmosphere, at a 1:1.05 molecular ratio:Ba+S+heat→BaS
Barium sulfide is prepared commercially by heating barite (BaO) with coal or petroleum coke in a rotary kiln at 1000°C to 1250°C in an oxygen-free atmosphere. The product, black ash, is a gray or black powder containing carbonaceous impurities and unreacted barite. Barium sulfide is separated from impurities by extraction with hot water and filtration. Barium sulfide may also be made by high temperature reduction of barium sulfate with methane.
생산 방법
Barium sulfide is grayish-white solid, formed by heating barium sulfate and carbon, reactive with H2O to form barium hydrosulfide, Ba(SH)2, solution. The latter is also made by saturation of barium hydroxide solution with H2S. Barium polysulfides are formed by boiling barium hydrosulfide with sulfur.일반 설명
Barium sulfide (BaS) is an inorganic compound that is used as a precursor in the production of barium compounds. These compounds can be used in a variety of applications, such as ceramics, luminous paints, additives, and flame retardants.위험도
Highly toxic by ingestion (see Barium).Safety Profile
A poison. Flammable by spontaneous chemical reaction, air, moisture, or acid fumes may cause it to ignite. For explosion and disaster hazards, see SULFIDES. To fight fire, use CO2, dry chemical. Reacts violently with phosphorus(V) oxide. Mxtures with lead dioxide, potassium chlorate, or potassium nitrite explode when heated. Incompatible with Cl2O, Ca(NO3)2, r(NO3)2, Ca(ClO3)2, Sr(ClO3)2, (ClO3)2. See also BARIUM COMPOUNDS (soluble) and SULFIDES.황화 바륨 준비 용품 및 원자재
원자재
준비 용품
황화 바륨 공급 업체
글로벌( 127)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| City Chemical LLC | 2039322489 |
sales@citychemical.com | United States | 168 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| TopScience Biochemical | 00852-68527855 |
info@itopbiochem.com | China Hong Kong | 902 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; |
factory@coreychem.com | China | 29826 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 |
dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 |
info@dycnchem.com | CHINA | 52867 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 |
alfa4@alfachem.cn | China | 12755 | 58 |
황화 바륨 관련 검색:
수산화 바륨 황화 수소 황산바륨
ASINEX-REAG BAS 03334576
ASINEX-REAG BAS 03293533
ASINEX-REAG BAS 03220611
ASINEX-REAG BAS 03752783
ASINEX-REAG BAS 03320425
ASINEX-REAG BAS 03220286
ASINEX-REAG BAS 03183701
ASINEX-REAG BAS 03213200
ASINEX-REAG BAS 03375399
ASINEX-REAG BAS 03439166
ASINEX-REAG BAS 03183422
ASINEX-REAG BAS 03107340
ASINEX-REAG BAS 03220609
ASINEX-REAG BAS 03559242
ASINEX-REAG BAS 00004210